Abstract
Background and Aim: Non-Alcoholic Fatty Liver Disease (NAFLD) is the commonest cause of chronic liver disease and is a leading cause of liver transplantation in the United States, with no approved medication to halt or reverse its progression. Recent animal-model prospective trial-suggested that drug Vedolizumab leads to improvement and reversal in the NAFLD-related metabolic derangements. Vedolizumab is an α4β7 integrin-inhibitor that is approved for use in IBD patients. Our study aims to understand Vedolizumab's impact on the course of NAFLD in inflammatory bowel disease (IBD) patients.
Methods: We conducted a retrospective cohort analysis of 158 subjects with NAFLD who received Vedolizumab at Cleveland Clinic Foundation (CCF). One cohort of 79 patients with NAFLD who received Vedolizumab were matched with control group of 79 patients. We determined the primary outcome as the response to Vedolizumab measured as Fibrosis-4 (Fib-4) regression to <1.3 points after one year of treatment.
Results: We observed that there was no statistically significant difference response (p= 0.576), progression of the disease (p= 1.000) or change in the number of cirrhosis decompensation episodes (in those with NAFLD cirrhosis) among Vedolizumab recipients.
Conclusions: In this retrospective cohort analysis, and unlike in the previous animal model, Vedolizumab was not associated with statistically significant improvement or progression in the Fib-4 score after one year of treatment, and among those with NAFLD-cirrhosis, there was no statistical difference in the complication rates.
Keywords
Non-Alcoholic Fatty Liver Disease (NAFLD), Vedolizumab, α4β7 integrin-inhibitor, Fib-4 score
Introduction
Non-Alcoholic Fatty Liver Disease (NAFLD) is the commonest cause of chronic liver disease and is a leading cause of liver transplantation in the United States, with no approved medication to halt or reverse its progression [1,2]. Patients with inflammatory bowel disease (IBD) often have a higher risk of incidence and prevalence of NAFLD, essentially because inflammation plays a key role in the pathogenesis of NAFLD. A recent animal-model prospective trial suggested that inhibiting integrin-mediated CD4 T cell recruitment leads to improvement and reversal in the NAFLD-related metabolic derangements [3]. Vedolizumab is an α4β7 integrin-inhibitor that is a gut-selective antibody that selectively prevents the infiltration of leucocytes into the gastrointestinal submucosa [4]. Vedolizumab has been previously approved for the treatment of active ulcerative colitis and Crohn's disease patients [5-7]. Unfortunately, there are no FDA-approved medications available for treating NAFLD. This study aims to evaluate Vedolizumab's influence on NAFLD in IBD patients.
Methods
A retrospective cohort analysis of all subjects with NAFLD who received Vedolizumab at Cleveland Clinic Foundation (CCF) was conducted. Institutional Review Board approval at CCF was obtained for this study.
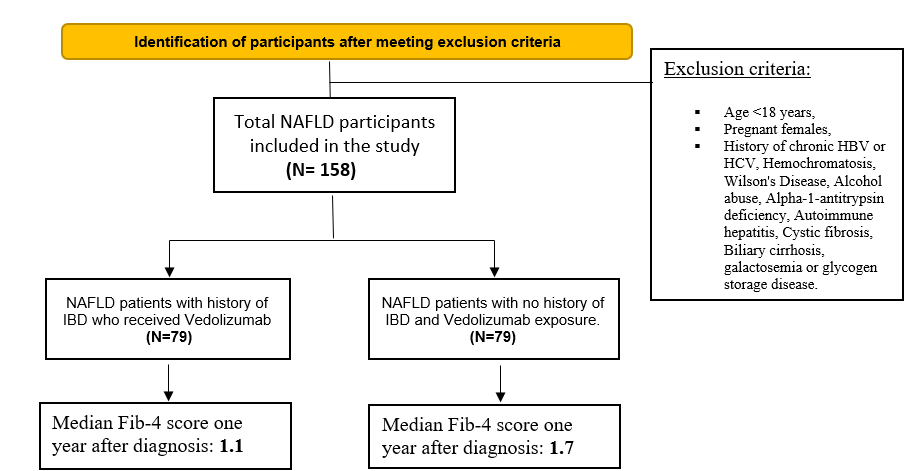
Cases of adults (above the age of 18 years) diagnosed with IBD who received Vedolizumab with a history of NAFLD at any lifetime point lifetime were included. A matching group of patients with NAFLD and no history of IBD/ Vedolizumab exposure was later created. We excluded patients with age <18 years, pregnant females, history of chronic HBV or HCV, Hemochromatosis, Wilson's Disease, Alcohol abuse, Alpha-1-antitrypsin deficiency, Autoimmune hepatitis, Cystic fibrosis, Biliary cirrhosis, galactosemia or glycogen storage disease. Patient Selection and Exclusion Criteria are shown in Figure 1.
Figure 1. Patient Selection and Exclusion Criteria.
We determined the primary outcome as the response to Vedolizumab measured as Fibrosis-4 (Fib-4) regression to <1.3 points after one year of treatment [8]. FIB-4 score and transient elastography may be used as alternatives to liver biopsy for fibrosis staging and patient follow-up. FIB-4 threshold of 1.3 was acceptable for excluding the presence of advanced fibrosis [9]. The secondary outcomes were the progression of the disease, which was defined as Fib-4 rise to >1.3 points, and a reduction in the number of decompensated cirrhosis episodes among those with NAFLD cirrhosis. FIB-4 has been shown to be a prognostic marker of liver-related outcomes in patients with NAFLD [10]. Studies have shown a high FIB-4 ≥ 2.67 as a strong predictor of both all-cause mortality and liver-related adverse outcomes independently of the baseline diagnostic group and common risk factors.
Appropriate weights were applied for all analyses using Stata version 17 (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC.). Chi-Square test and student t-tests were used for statistical analysis using SPSS (SPSS Inc, Chicago, Illinois, United States). P-value <0.05 was considered significant. Results were reported as mean ± standard deviation (SD) for quantitative variables and percentages for categorical variables. Statistical significance was based on two-sided design-based tests evaluated at α=0.05.
Results
A total of 158 patients with diagnoses of coexisting IBD and NAFLD in the Cleveland Clinic system were included in the final analysis. 79 patients with NAFLD have also received Vedolizumab, in contrast to the 79 matching control group. The baseline characteristics of the study population is shown in Table 1.
|
|
All patients N= 158 |
Vedolizumab N= 79 (%) |
No Vedolizumab N=79 (%) |
P-value |
|
Age, years |
62 (52-71) |
54 (39-66) |
66 (60-74) |
0.000 |
|
Male gender |
75 (47) |
40 (51) |
35 (44) |
0.426 |
|
Past medical history |
|
|
|
|
|
Hypertension |
98 (62) |
40 (51) |
58 (73) |
0.003 |
|
Type 2 diabetes |
66 (42) |
27 (35) |
39 (49) |
0.061 |
|
History of cirrhosis |
21 (13) |
13 (16) |
8 (10) |
0.241 |
|
Dyslipidemia |
80 (51) |
35 (45) |
45 (58) |
0.109 |
|
Hypothyroid |
28 (18) |
11 (14) |
17 (22) |
0.211 |
|
Laboratory results |
|
|
|
|
|
Median AST at diagnosis |
27 (20-40) |
23 (17-34) |
29 (22-48) |
0.003 |
|
Median ALT at diagnosis |
28 (19-44) |
25 (18-38) |
30 (21-52) |
0.045 |
|
Median AST one year after diagnosis |
27 (21-36) |
27 (19-36) |
28 (22-35) |
0.370 |
|
Median ALT one year after diagnosis |
28 (17-42) |
27 (17-42) |
29 (17-42) |
0.450 |
|
Median Fib-4 score at diagnosis |
1.4 (0.9-2.0) |
1.1 (0.6-1.8) |
1.6 (1.2-2.2) |
0.000 |
|
Median Fib-4 score one year after diagnosis |
1.4 (0.8-2.1) |
1.1 (0.6-1.9) |
1.7 (1.2-2.1) |
0.0001 |
|
Response |
14 (9) |
6 (8) |
8 (10) |
0.576 |
|
Progression |
20 (13) |
10 (13) |
10 (13) |
1.000 |
|
Complications |
|
|
|
|
|
Hepatic encephalopathy |
4 (3) |
3 (4) |
1 (1) |
0.311 |
|
Variceal bleeding |
3 (2) |
1 (1) |
2 (3) |
0.560 |
|
Ascites |
6 (4) |
3 (4) |
3 (4) |
1.000 |
|
HRS |
2 (1) |
1 (1) |
1 (1) |
1.000 |
The primary outcome was the response to Vedolizumab measured as Fibrosis-4 (Fib-4) regression to <1.3 points after one year of treatment in both groups. Median Fib-4 score one year after diagnosis in Vedolizumab group was 1.1 (0.6-1.9) vs non-vedolizumab group 1.7 (1.2-2.1).
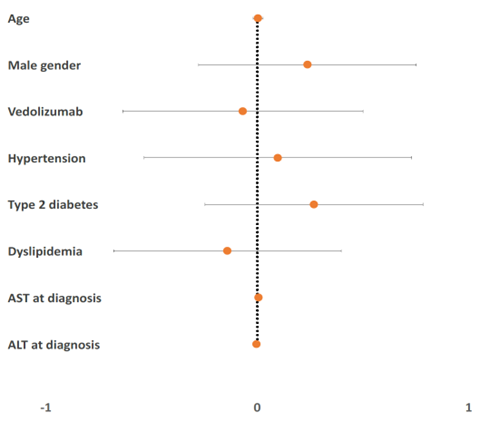
There was no statistically significant difference in response (p= 0.576), progression of the disease (p= 1.000), or change in the number of cirrhosis decompensation episodes (in those with NAFLD cirrhosis) among Vedolizumab recipients. The predictors for response are shown in Figure 2.
Figure 2. Predictors of Positive Response.
Discussion
In our retrospective cohort analysis, we found that Vedolizumab was not associated with statistically significant improvement or progression in the Fib-4 score after one year of treatment, and among those with NAFLD-cirrhosis, there was no statistical difference in the complication rates.
NAFLD is a spectrum of liver disease characterized by the presence of extra fat in liver cells without any linkage to alcohol consumption. Over the last couple of decades, globally, NAFLD has emerged as one of the most common causes of chronic liver disease. A large part of the cause is the global epidemic of obesity and diabetes. It is estimated that the overall prevalence of NAFLD in North America is 24% [11]. Currently, there are no specific FDA-approved treatments for this disease. Hence there is a dire need for appropriate therapeutic agents. For over a decade, certain lifestyle modifications have been the cornerstone for treating NAFLD. Some of these widely practiced lifestyle changes include physical activity, caloric restriction, and time-restricted feeding. There have been a number of randomized controlled trials on pharmacological agents that have shown histological improvements in the NASH/NAFLD spectrum.
Indirect pharmacological therapeutic agents have been used in cases with advanced fibrosis. These agents primarily aim to curb the inflammatory response mount by steatosis. Insulin sensitizers, including thiazolidinediones [12] and Peroxisome proliferator-activated receptors (PPARs) [13], have been studied extensively in the past, but their role and long-term benefits have remained controversial. Pentoxifylline , a non-selective phosphodiesterase inhibitor, is another pharmacological agent which showed some promising results in animal studies models but failed to replicate the same results in human subjects [14]. As per ongoing the FLINT trial [15] and REGENERATE Trial [16], which is a double-blinded randomized control trial that studies the role of obeticholic acid as an anti-inflammatory agent. The earlier phase of this trial has shown some promising results with improvement in liver histology and halting hepatic fibrosis. However, the resolution of NASH was not seen in any of the subjects. Up to this date, the most remarkable study done in the arena is the PIVENS trial [17], which compared Vitamin E role with pioglitazone and placebo for the Treatment of Nondiabetic Patients With NASH.
Several trials conducted in the past failed to show a therapeutic effect for reversing if not halting inflammatory process in NAFLD/NASH axis. A cutting-edge animal model-based trial established that a mucosal vascular addressin cell adhesion molecule 1 (α4β7/MAdCAM-1) plays a vital role in NASH development through colonic and hepatic CD4 T cell recruitment. Vedolizumab is an α4β7 integrin-inhibitor that is a gut-selective antibody that selectively prevents the infiltration of leucocytes into the gastrointestinal submucosa [4]. We conducted a first retrospective cohort analysis on human subjects to speculate on the anti-inflammatory response of Vedolizumab drug on the NASH/NAFLD axis. We created a retrospective database and did a cohort analysis to compare 158 patients with commitment diagnoses of NAFLD & IBD. We fail to show a statistically significant difference in response (p=0.576), progression of the disease (p=1.000), or change in the number of cirrhosis decompensation episodes (in those with NAFLD cirrhosis) among Vedolizumab recipients.
Though our results are negative, and we are discouraged that Vedolizumab doesn't demonstrate, our study opens a great opportunity door to reassess similar pharmacological therapies for the management of NAFLD. Perhaps the hepatology community needs better studies with a better sample size to show statistical significance. While there is no FDA-approved medication for NAFLD/NASH, dietary and lifestyle intervention is the mainstay of treatment.
Conclusion
In conclusion, our retrospective cohort analysis showed that Vedolizumab did not have a significant impact on the Fib-4 score or complication rates in NAFLD-cirrhosis patients after one year of treatment. NAFLD is a prevalent cause of chronic liver disease, and while lifestyle modifications are the mainstay of treatment, various pharmacological agents have been studied with controversial long-term benefits. Our study highlights the need for further research into pharmacological therapies for NAFLD management, as several trials have failed to demonstrate a therapeutic effect. Ultimately, larger studies are necessary to provide statistical significance and supplement current dietary and lifestyle interventions for treating NAFLD/NASH.
Authors’ Contribution
Prabhat Kumar: First Co-author and manuscripts writing.
Ashraf Almomani: First Co- Author and scientific review.
Almaza Albakri, Motasem Alkhayyat, Tariq Kewan: Biostatistical analysis.
Antoine Boustany, Somtochukwu Onwuzo: Data collection.
Prabhat Kumar, Eduard Krishtopaytis, Dana Alshaikh: Literature review.
Disclosure
There are no potential conflicts (financial, professional, or personal) to disclose by all the authors.
References
2. Masuoka HC, Chalasani N. Nonalcoholic fatty liver disease: an emerging threat to obese and diabetic individuals. Ann N Y Acad Sci. 2013 Apr;1281(1):106-22.
3. Rai RP, Liu Y, Iyer SS, Liu S, Gupta B, Desai C, et al. Blocking integrin α4β7-mediated CD4 T cell recruitment to the intestine and liver protects mice from western diet-induced non-alcoholic steatohepatitis. J Hepatol. 2020 Nov;73(5):1013-1022.
4. Mosli MH, Rivera-Nieves J, Feagan BG. T-cell trafficking and anti-adhesion strategies in inflammatory bowel disease: current and future prospects. Drugs 2014;74:297-311.
5. Smith MA, Mohammad RA. Vedolizumab: an α4β7 integrin inhibitor for inflammatory bowel diseases. Ann Pharmacother. 2014 Dec;48(12):1629-35.
6. Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699-710.
7. Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, et al. Vedolizumab as induction and 5 maintenance therapy for Crohn's disease. N Engl J Med 2013;369:711–21.
8. Long MT, Noureddin M, Lim JK. AGA Clinical Practice Update: Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Lean Individuals: Expert Review. Gastroenterology. 2022 Sep;163(3):764-774.e1.
9. Davyduke T, Tandon P, Al-Karaghouli M, Abraldes JG, Ma MM. Impact of Implementing a "FIB-4 First" Strategy on a Pathway for Patients With NAFLD Referred From Primary Care. Hepatol Commun. 2019 Jul 29;3(10):1322-1333.
10. Vieira Barbosa J, Milligan S, Frick A, Broestl J, Younossi Z, Afdhal NH, et al. Fibrosis-4 Index as an Independent Predictor of Mortality and Liver-Related Outcomes in NAFLD. Hepatol Commun. 2022 Apr;6(4):765-779.
11. Arshad T, Golabi P, Henry L, Younossi ZM. Epidemiology of Non-alcoholic Fatty Liver Disease in North America. Curr Pharm Des. 2020;26(10):993-997.
12. Colca JR, McDonald WG, Adams WJ: MSDC-0602K, a metabolic modulator directed at the core pathology of non-alcoholic steatohepatitis. Expert Opin Investig Drugs. 2018;27(7):631-636.
13. 13
14. Zein CO, Yerian LM, Gogate P, Lopez R, Kirwan JP, Feldstein AE, McCullough AJ. McCullough: Pentoxifylline improves non-alcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology, 2011;54(5):1610-9.
15. Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. Network: Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non- alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo- controlled trial. Lancet. 385(9972):956- 65
16. Ratziu V, Sanyal AJ, Loomba R, Rinella M, Harrison S, Anstee QM, et al. Younossi: REGENERATE: Design of a pivotal, randomised, phase 3 study evaluating the safety and efficacy of obeticholic acid in patients with fibrosis due to non-alcoholic steatohepatitis. Contemp Clin Trials. 2019;84:105803.
17. Gawrieh S, Wilson LA, Yates KP, Cummings OW, Vilar-Gomez E, Ajmera V, Kowdley KV, et al. Relationship of ELF and PIIINP With Liver Histology and Response to Vitamin E or Pioglitazone in the PIVENS Trial. Hepatol Commun. 2021 Feb 5;5(5):786-797.