Abstract
Purpose: The number of years spent living with disability and the overall mortality for chronic neurological conditions is increasing worldwide. Cranial nerve non-invasive neuromodulation (CN-NINM) via translingual nerve stimulation (TLNS) is a new technology developed as an adjunct to neurological rehabilitation to improve outcomes for those with neurological conditions. This scoping review will explicate the evidence available for the use of TLNS in neurological populations in order to inform future research and healthcare policies.
Materials and Methods: Academic databases MEDLINE, AMED, CINAHL, Embase and Web of Science, and gray literature databases DuckDuckGo, ProQuest and Google were searched for studies published from 2000-2021. Two independent assessors screened the titles and abstracts and included full texts that met a-priori inclusion criteria. Data was extracted and the results were summarized in tables.
Results: Of the 11,799 articles retrieved from the primary search, 40 studies fulfilled inclusion criteria. The diagnostic groups who received therapy with TLNS included persons with traumatic brain injury, multiple sclerosis, Parkinson’s disease and spinal cord injury. Most studies used the PoNS device in conjunction with a rehabilitation program to improve functional outcomes such as balance and gait. No serious adverse events were reported.
Conclusion: The findings suggest that TLNS is a feasible modality that can be incorporated into home-based programs. If paired with an individualized rehabilitation program, it has the potential to produce lasting neuroplastic change that improves balance and gait. However further research on which populations, including clinical indicators, is indicated for TLNS and the optimal parameters are required.
Keywords
Neurorehabilitation, Neuromodulation, Translingual Neurostimulation, PoNSTM Device, Cranial Nerve
Introduction
Chronic neurological disorders are the leading cause of disability worldwide and their burden of death and disability is increasing [1,2]. Of all chronic neurological disorders, stroke, multiple sclerosis (MS) and traumatic brain injury (TBI) are the three largest contributors to disability and mortality [1,3]. Despite the incidence of chronic neurological conditions declining from 1990 to 2015, time spent living with a disability, and mortality rates from chronic neurological conditions has been increasing [1]. These findings suggest that people with chronic neurological conditions are living longer with persistent disability [1].
Recent literature has explored the burden of disability for persons with neurological conditions [4-6]. People with chronic neurological conditions have a wide range of gait dysfunctions including, but not limited to, deficits in gait initiation, movement fluidity, and gait speed [4]. In addition, balance impairments demonstrating increased postural sway and instability are a significant problem for the neurological population [4]. As a result, older adults living with neurological disorders are a significant higher falls risk compared to older neurotypical adults [5]. For example, Coote et al. reported that 71% of older adults with MS sustained a fall compared to 41% of age-matched controls over a six-month period [6]. Furthermore, falls in persons with MS were more likely to cause serious injury [6]. Similarly, a systematic review by Lai et al. reported approximately 73% of individuals who have sustained a stroke will experience a fall in the first six months following hospital discharge [5]. The development of new effective rehabilitation interventions to ameliorate persistent neurological-related movement disabilities, such as balance and gait, are required to improve the lives of persons with neurological conditions and for improved sustainability of healthcare systems [1].
Cranial-nerve non-invasive neuromodulation (CN-NINM) is a new and emerging technology that aims to facilitate neuroplasticity in individuals living with chronic neurological conditions [7-12]. CN-NINM technology utilizes translingual neurostimulation (TLNS) via a portable neuromodulation stimulation (PoNSTM) device [13-16]. When combined with targeted physical rehabilitation, the PoNSTM device has been reported to improve long-standing balance and gait deficits in persons with chronic neurological conditions [13,14,16].
The PoNSTM device is a portable device with an electrode array placed on the anterior dorsal surface of the tongue [10,13,17], providing a natural form of stimulation to cranial nerves V (trigeminal nerve) and VII (facial nerve) [16,18-20]. Additional cranial nerves (CN) that have been reported to be stimulated include the glossopharyngeal (CN-IX), vagal (CN-X), and hypoglossal (CN-XII) nerves [11,18]. Stimulation of the CNs subsequently stimulate targeted areas in the brainstem and cerebellum through the lingual branch of CN-V and chorda tympani branch of CN-VII [18]. It is hypothesized that stimulation of these structures modulates activity of corresponding nuclei of the brainstem in the sensory and spinal nuclei of the trigeminal nuclei complex and the caudal portion of the solitary nucleus tract [16]. In turn, connected regions are also modulated, stimulating corresponding neural networks and subsequent neurotransmitter release [16,20,21]. When combined with physiotherapy, this potentiates long-term neuroplastic change [10].
Randomized and non-randomized control trials, as well as small sample research designs have revealed promising results for CN-NINM using TLNS in chronic neurological conditions [18]. For example, improvements in chronic balance deficits, falls, and gait have been demonstrated in individuals living with moderate TBI [7,8,14,15], stroke [15,22-25], MS [26] and Parkinson’s disease (PD) [26], as well as in persons with refractory balance deficits [9].
The purpose of this scoping review is to explicate the evidence available for the use of TLNS in neurological populations. A preliminary search of PROPSERO, MEDLINE, the Cochrane Database of Systematic Reviews and the JBI Evidence Synthesis was conducted on February 14, 2021, at which time no current or underway scoping reviews were identified. This will be the first scoping review addressing TLNS literature to inform future research and healthcare policies. The protocol for this scoping review was specified in advance and published in JMIR Research Protocols [27-30].
Objectives
The following objectives were addressed by this review:
- What is CN-NINM, and how is CN-NINM being delivered?
- What are typical parameters used in TLNS?
- Which conditions or clinical symptoms have evidence supporting the use of TLNS?
- How is TLNS being incorporated into neurological rehabilitation?
- What has previous research shown on the effects of TLNS in neurorehabilitation?
- What are the current gaps in the evidence base and the implications for rehabilitation science?
Methods
This scoping review followed the Joanna Briggs Institute (JBI) methodology to map the available literature on CN-NINM [31]. The study was designed and conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Review (PRIMSA-ScR) [32]. The protocol was registered on Open Sciences Framework and published in JMIR research protocols [30,33].
Inclusion/Exclusion criteria
Eligibility criteria were established a priori utilizing the population, concept, context framework [31]. Studies were selected in accordance with the following criteria.
Population: Studies were included that involved adults with neurological conditions (≥ 18 years of age). Conditions of interest included, but were not limited to, stroke, mild TBI including concussion, moderate TBI, severe TBI, and MS. Given that neuromodulation interventions for depression are typically invasive in nature and not via TLNS, depression was excluded from the current study. Trigeminal neuralgia was also excluded, as our search retrieved an overwhelming number of articles pertaining to the condition and TLNS is rarely used in this population.
Concept: We explored the concepts of TLNS, the PoNSTM device and CN-NINM. It excluded other forms of invasive and non-invasive brain stimulation such as, transcranial magnetic stimulation, functional electrical stimulation, transcranial electrical stimulation techniques and deep brain stimulation. Studies pertaining to Vagus nerve stimulation were excluded given that this type of nerve stimulation primarily involves an implantable device.
Context: We considered studies conducted in any context and geographical location to widely explore the application of CN-NINM in neurological rehabilitation.
Types of sources: Limited restrictions were placed on the types of sources included in this review. This scoping review considered studies with qualitative, quantitative, and mixed methods design. Furthermore, systematic reviews and gray literature including non-research articles, opinion papers, texts, as well as documents from relevant websites were considered. Posters and unpublished theses were considered gray literature. Studies in French and English published since 2000 were included. This time frame was deemed appropriate as TLNS is a new technology that emerged from the sensory substitution research performed in the early 1990’s at the Tactile Communication and Neurorehabilitation Laboratory at the University of Wisconsin-Madison [16,34]. Abstracts were excluded due to resource constraints and the limited information offered given the brevity of abstracts.
Search strategy
An initial limited search of MEDLINE and CINAHL was undertaken to identify articles on the topic. Text words contained in the titles and abstracts of relevant articles found in the initial limited search, along with key concepts and Medical Subject Heading suggested by an academic librarian at McMaster University were used to develop the search strategy. Comprehensive search strategies were developed in collaboration with the librarian. These search strategies were developed for five databases: MEDLINE, AMED, CINAHL, EMBASE and Web of Science. The detailed search strategy is provided in Appendix 1.
Sources of unpublished studies and gray literature included ProQuest, DuckDuckGo and Google. Consistent terms were used for each browser and the date and time as well as the results of the search were documented (Appendix 2). For the gray literature search, the first five pages of results from each respective database were screened. If a document met the inclusion criteria a backward and forward reference search was performed to identify other relevant documents. The research team also contacted and received gray literature from author Dr. Yuri Danilov and Helius Medical Technologies.
Hand-searching of all reference lists of included studies was undertaken to identify additional studies of relevance.
Selection process
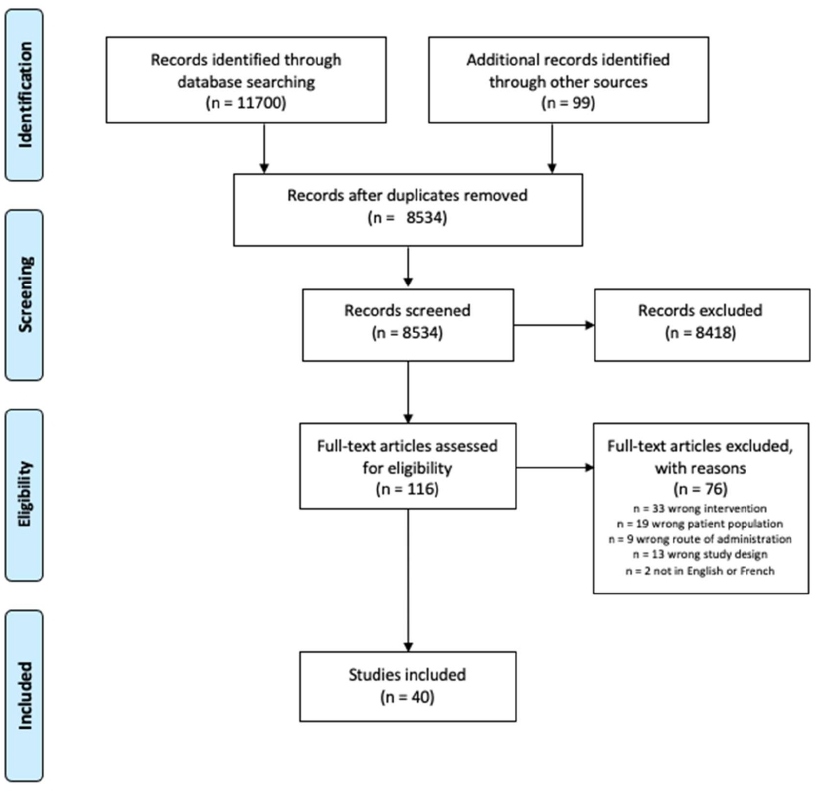
All identified records were collated and imported to Zotero reference management software (Center for History and New Media, George Mason University) and duplicates were removed. All research team members screened the first ten citations of the initial MEDLINE search to ensure consistency in application of the a priori inclusion and exclusion criteria and researcher agreement. Any discrepancies were discussed and resolved collectively by the research team. The titles and abstracts of the remaining articles were screened by groups of two researchers and any disagreements were resolved between the two researchers, or in the case that an agreement was not reached, senior researchers were consulted. Full text of potentially relevant papers was retrieved and imported into Covidence screening and data extraction tool (Veritas Health Innovation, Melbourne, Australia). Full text reviews were performed by groups of two researchers. The results of the search and reasons for exclusion were reported in full and presented in a PRISMA-ScR flow diagram (Figure 1).
Figure 1: Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram for this review.
Data extraction
The data extraction table proposed in the protocol paper was trialed with two resources to ensure all relevant data were extracted [30]. Minor revisions were made such as extracting funding information as well as description of mechanism of action, type of device used and indications for use (Appendix 3).
The data extraction process was completed by groups of two researchers and to ensure accurate data collection, the extracted data was compared and discrepancies were resolved by consensus.
Results
A total of 11,700 records were retrieved from the five databases selected for this study. Additionally, 99 articles were retrieved from our gray literature search. Of these, 40 studies met all inclusion criteria and were included in the scoping review. Details of the study selection process are outlined in Figure 1. The 40 studies consisted of one systematic review [11], five RCTs [21,35-38], five non-RCTs [9,12,17,39,40], two pilot studies [13,41], six case reports [8,19,20,22,25,42] and nine articles on conceptual literature [10,16,23,43-48]. Furthermore, 12 gray literature sources were obtained which included posters, poster presentations and facts sheets [14,15,24,26,49-56]. Of the included articles, seven were published in Canada [9,14,17,22,35,42,46], three in Australia [13,19,20], 29 from United States [8,10,11,13,15,16,21,23-26,36-41,43-45,47,49-56] and one from Ireland [48].
The systematic review and randomized controlled trials are described in Table 1. The systematic review comprised five studies. Three of the studies were included in our scoping review [38-40], the remaining two studies were excluded as they did not meet our inclusion criteria [57,58]. Two of the five studies [38,58] had usable outcomes measures that could be combined into a meta-analysis (MA). The MA reported improvements in balance and gait following CN-NINM in a variety of neurological conditions, however, because one of the studies [58] was not included in our study (due to use of an alternative device (BrainPort balance device)), we cannot consider the results in our scoping review.
| Citation | Population | Intervention | Control | Frequency of intervention | Outcome Measures & Intervals | Results | Conclusion | Funding |
|---|---|---|---|---|---|---|---|---|
| Systematic Review | ||||||||
| Papa et al. (2014) | N= 5 studies [38-40,57,58] Chronic balance dysfunction | CN-NINM + Balance | N/A | N/A | DGI DHI ABC SOT fMRI |
DGI, ABC, DHI, improved significantly with CN-NINM. Benefits seen were long lasting. | CN-NINM has demonstrated balance and sensory motor improvements in peripheral and central conditions. | Not stated |
| Randomized Controlled Trials | ||||||||
| Hou et al. (2020) | N=9 TBI | High frequency CN-NINM+ balance, gait and exercise training | Low frequency CN-NINM+ balance, gait and exercise training | 2x/day, 2 wks |
SOT DGI sMRI Assessment: Baseline, weeks 2, 5, 8, 11, 14, 17, 20, 23, 26 |
Balance, Gait Significant improvements seen in both groups. No between group differences. Both groups found an increase in GMV across 11 regions. |
Either low or high frequency can be used for people with TBI to improve balance and gait. Can be at used safely at home. | Helius Medical Technologies and the National Center for Research Resources. |
| Ptito et al. (2020) | N=122 TBI |
HF CN-NINM + PT | LF CN-NINM + PT | 100-120 mins/day, 2 wks in lab + 3 wks at home |
SOT Frequency of falls 6MWT QLMI SQI Assessment: Baseline, 2wk, 5 wk |
SOT, DGI, number of headaches and fall improved significantly in both groups. No between group differences. | CN-NINM+ PT is promising and safe for chronic balance deficits. | US Army Medical Research and Materiel Command (USAMRMC) and Helius Medical Inc. |
| Tyler et al. (2019) | N=44 TBI |
HF CN-NINM + PT | LF CN- NINM + PT | 3x/day, 14 wks Stage 1: 2 wks, supervised training Stage 2: 12 wks, 6 days at home, 1 in clinic Stage 3: 12 wks, no treatment only regular daily activity |
SOT 6MWT DGI NSI BSI-18 PSQI Assessment: Baseline, weeks 2,5,14,26 |
Both HF and LF groups significantly improved in gait. No between group difference. | Both High frequency and low frequency improve balance in TBI. | University of Wisconsin Foundation, Department of Defense, and Helius supported the editorial development of the manuscript. |
| Tyler et al. (2014) | N=20 MS | CN-NINM + exercise and relaxation | CN-NINM device at stimulation level not perceivable + exercise and relaxation | 2x/day. 2 weeks in lab and 12 wks at home 20 mins no device + exercice, 20 mins PoNS + exercice, 20 mins PoNS + relaxation |
DGI EDSS Assessment: baseline, 2,6,10,14wks |
DGI: improved statistically in intervention group at 6 wks and 14 wks, average 7.95. No significant improvements at any point in control. | CN-NINM combined with PT can reduce symptoms of gait dysfunction in MS. | University of Wisconsin foundation. |
| Wildenberg et al. (2011) | N=12 Chronic balance dysfunction | CN-NINM + Standing balance EC |
9 healthy controls | 2x/ day Days 1-4 1x/day Day 5 20 mins |
DGI ABC MRI Assessment: Pre-Post |
Significant improvements in DGI, ABC, and DHI in the active group. | CN-NINM can improve gait, self-perception of functional mobility, and dizziness in people with chronic balance impairment. | National Institute of Diabetes and Digestive and Kidney Diseases, the Clinical and Translational Science Award program of the National Center for Research Resources, National Institute of Health, and UW-I&EDR. |
| MS: Multiple Sclerosis; TBI: Traumatic Brain Injury; SOT: Sensory Organization Test; DGI: Dynamic Gait Index; sMRI: structural Magnetic Resonance Imaging; 6MWT: 6 Minute Walk Test; QLMI: Quality of Life Measure; SQI: Sleep Quality Index; NSI: Neurobehavior Symptom Inventory; BSI-18: Brief Symptom Inventory -18; PSQI: Pittsburgh Sleep Quality Index; EDSS: Expanded Disability Status Scale; ABC: Activity Specific Balance Confidence Scale; fMRI: Functional Magnetic Resonance Imaging; EC: Eyes closed; DHI: Dizziness handicap index; GMV: Grey Matter Volume | ||||||||
Five RCTs reported on the effects of the PoNSTM device combined with PT, or an individualized exercise and balance training program, on gait and balance in individuals with MS [37], TBI [21,35,36] and chronic balance dysfunction [38]. In each study, the intervention group was compared to a control group who were administered a sham treatment using a low frequency stimulation (below stimulation threshold) PoNSTM device [21,35-38]. Two of the studies reported improvements in gait [37,38] and one reported improvement in balance [38]. Three studies reported no significant between group differences in balance and gait [21,35,36]. Additionally, one study found that there was no between group differences for high or low frequency groups in grey matter volume across 11 different brain regions [21].
Five of the 40 articles were non-randomized controlled trials [9,12,17,39,40] and two were pilot studies [13,41] (Table 2) all of which used the PoNSTM device. These studies investigated stroke, MS, TBI, PD, chronic balance dysfunction, and neurotypical adults. Two studies used fMRI to map brain activity during stimulation [12,40]. Two studies [12,40] reported on the effects of CN-NINM on optic flow, with only one study reporting an upregulation [40]. One study used EEG to measure brain activity and found an increase in alpha and theta activity [8]. Balance was reported to improve in three studies [13,39,41]. Additionally, two studies investigated cognition, both of which reported improvements [17,41]. Finally, Danilov (2013) reported improvements in word processing [41].
|
Citation |
Population |
Intervention |
Control |
Frequency of intervention |
Outcome Measures and intervals |
Results |
Conclusion |
Funding |
|---|---|---|---|---|---|---|---|---|
|
Frehlick et al. (2019) Counterbalanced cross-over |
N=20 Neurotypical adults |
20 min resting+ 20 min with CN-NINM + 20 min resting Group 1: HF 1 week wash out then LF Group 2: LF 1 week wash out then HF |
|
Day 1: LF or HF Rx
|
EEG Assessment: Baseline and 1 wk |
Increased main effect of time on alpha brain activity (p=0.012) in high frequency group. Group 1 increased alpha and theta activity (p=0,007). |
CN-NINM significantly increases brain activity at rest. |
Funded Helius medical technologies. |
|
Galea et al. (2017) Pilot Study
|
N= 10 Stroke |
CN-NINM+ Balance and gait training |
Balance and gait training |
90 mins @ 2x/day,
|
MiniBEST Assessment: |
MiniBEST: Reported OM Fall cut-off is 17.5pts. |
CN-NINM + PT was more effective than high intensity PT alone in balance. |
Private philanthropic grant to the Royal Melbourne Hospital |
|
Leonard et al. (2017) Non-RCT |
N=14
|
CN-NINM + exercise and BAT
|
CN-NINM device at unperceivable stimulation level + exercise and BAT |
90 mins @ 2x/day,
|
MFIS Assessment: baseline + 2 wk intervals for 14 weeks |
MFIS, MSIS-29, BDI-11, DGI, and BAI: COGMED: SOT: Increased brain activation in active group.
|
CN-NINM + PT is effective in multitasking, vision, fine motor control and sleep. |
Helius Medical technologies under contract with McGill and the clinical Research Unit at the Montreal neurological institute. |
|
Danilov et al. (2013) Pilot study |
N=4 |
CN-NINM + flexibility and conditioning |
None |
2x/day, 5x/wk,
|
DGI Assessment: Pre-post |
DGI: SOT: Reported OM MCID is 5.0 pts Improved Word span, Word storage, |
CN-NINM + PT improved gait, cognition, balance and eye movements. |
University of |
|
Wildenberg et al. (2013) Non-RCT |
N= 21 Assessed at baseline and day 5 |
CN-NINM in chronic balance deficit individuals 20 mins |
CN-NINM in neurotypical individuals 20 mins |
2x/day, |
fMRI Assessment: during each session |
Upregulation in balance-impaired individual not seen following CN-NINM. |
CN-NINM can decrease the weighting of visual motion input into the system. |
CTSA program |
|
Wildenberg et al. (2011) Non-RCT |
N=18
|
CN-NINM
|
No treatment given |
20 mins @ 2x/day, |
fMRI SOT Assessment: Pre-post, day 10 |
SOT: improved 15.75 pts. Optic flow was activated in cortical structures and cerebellar structures. Optic flow decreased after stimulation in balance deficit participants. |
CN-NINM activated and leads to interaction between vestibular system and visual system. |
University of Wisconsin Foundation. |
|
Wildenber et al. (2010) Prospective Cohort |
N=21 12 chronic balance deficits 9 control |
CN-NINM + standing eyes closed in chronic balance deficit individuals 20 mins
|
CN-NINM + standing eyes closed in healthy individuals 20 mins |
2x/day, |
MATLAB for postural sway Assessment: |
Optic flow effect decreases after CN-NINM is stopped. |
CN-NINM decreased postural sway and decreased susceptibility to optic flow with 1 week of training. |
CSTA and UW-I&EDR. |
|
MS: Multiple Sclerosis; TBI: Traumatic Brain Injury; PD: Parkinson’s Disease; HF: High Frequency; LF: Low Frequency; MSIS-29: MS Impact Scale; SOT: Sensory Organization Test; DGI: Dynamic Gait Index; ABC: Activity Specific Balance Confidence Scale; fMRI: functional Magnetic Resonance Imaging; BRB: Brief Repeatable Battery or Neuropsychological Test; BDI-II: Beck’s Depression Inventory; MFIS: Modified Fatigue Impact Scale; TUG: Timed Up and GO; COPM: Canadian Occupational Performance measure; DASS: Depression Anxiety Stress Scale; COGLOG: Cognitive Log; EEG: Electroencephalogram; BAI: Beck Anxiety Inventory |
||||||||
Six of the 40 articles used a small sample research design of which one was a case report [22], and five were N-of-1 studies [8,19,20,25,42] (Table 3). The populations studied included SCI, TBI, spinocerebellar ataxia, posterior fossa craniectomy and stroke. Four studies found improvements in balance [19,20,22,42] and one study reported a reduction in postural sway [20]. Two studies reported improvements in gait [20,22]. Quality of life measures were investigated in three studies [8,22,25]. One study reported improvements in depression, memory, emotions, and hand dexterity [25], while another reported a reduction in post-traumatic stress disorder and terrors [8]. Furthermore, one assessed QoL via a variety of outcomes measures, however, did not report or comment on overall improvements of QoL, or lack thereof [22].
|
Citation |
Population |
Intervention |
Device |
Frequency of Intervention |
Outcomes Measures & Intervals |
Results |
Conclusions |
Funding |
|---|---|---|---|---|---|---|---|---|
|
Chisholm et al. (2014)
|
N=2 Incomplete SCI |
CN-NINM + balance and gait |
PoNS 19V pulse at 200Hz. Every 4th pulse was removed |
50 mins @ 5x/wk 12 wks in lab + 12 weeks home program
|
Static balance Assessment: |
Balance and Gait improved. QOL may have improved. |
Sensory tongue stimulation combined with task-specific training can enhance balance and gait functions in those with incomplete spinal cord injury. |
ICORD Seed Grant. |
|
D'Arcy et al. (2020)
|
N=1 TBI |
CN-NINM+ UE training and gait training |
PoNS |
2-3x day,
|
Timed stand FIST Assessment: baseline + 3 mthly for 1 yr |
Timed Stand increased to 2 mins min assist x 1. FIST: |
PT + PoNS leads to increased movement ability. |
NRC and NSERC. |
|
Fickling
|
N= 1 |
CN-NINM + PT |
PoNS |
1 hr @ 2x/day 14 wks
|
EEG Assessment: Baseline, 7 &14 wks |
Decreased night terrors and PTSD symptoms. |
CN-NINM may provide benefits for TBI in respect to attention and cognition. |
NRC and NSERC. |
|
Bastani et al. 2018
|
N=1 Spinocerebellar ataxia |
CN-NINM + High intensity PT |
PoNS |
2 x 1.5 hrs/ day 2 wks
|
MiniBEST Assessment: Pre-post |
MiniBEST: |
CN-NINM + high-intensity PT may be beneficial in spinocerebellar ataxia. |
Not stated. |
|
Lizama et al. (2018)
|
N=1 Posterior fossa craniotomy |
CN-NINM + balance and gait, relaxation and medication |
PoNS |
90 mins @ 5x wks,
|
MiniBEST Assessment: Pre-post |
Improvement in MiniBEST, gait, postural sway and mean velocity. Reduced number of falls. |
PoNS combined with PT had a positive impact on balance and gait. No adverse events seen. |
Private Philanthropic Grant to the Royal Melbourne Hospital. |
|
Paltin et al (2017)
|
N=1 Stroke |
TLSN + physical therapy and target therapy |
PoNS |
2 wks in lab + |
SIS Assessment: baseline, and following each phase, every 2 months in phase 4. |
Strength improved +33.21%. Hand function improved +275%. ADL improved significantly. Emotion improved +17.1%. Memory and thinking +47.3%. RBANS decreased during withdrawal. |
Decrease in outcomes during withdrawal indicated a potentional dose-dependence effect. |
Not stated. |
|
TBI: Traumatic Brain Injury; MS: Multiple Sclerosis; SCI: Spinal Cord Injury; DGI: Dynamic Gait Index; DIP: Dysarthria Impact Profile; SOT: Sensory Organization Test; TUG: Timed Up and Go; SIS: Stroke Impact Scale; RBANS: Repeatable Battery for the Assessment of Neuropsychological Status; FIST: Function in sitting |
||||||||
Nine articles were conceptual literature comprising four literature reviews [10,43,47,48], one narrative review [46], and four book chapters (Table 4) [16,23,44,45]. The populations included in these publications were TBI, MS, SCI, Cerebral Palsy, refractory balance and gait disorders, PD, stroke, cerebellar degeneration, and posterior fossa syndrome. All 9 articles described PoNSTM as the device used for TLNS, to facilitate neuroplastic change. Six of the 9 articles referred directly to stimulation of the CN-V and CN-VII via TLNS [16,23,43,44,46,48] whilst three articles identified use of TLNS but did specifically indicate which nerves were being accessed [10,45,47]. Seven articles suggested that TLNS would be an appropriate adjunct therapy to augment and or enhance physical rehabilitation [16,23,43-46,48]. Eight articles reported improvements in balance and gait dysfunction [10,16,24,43,45-48] and 8 articles indicated that TLNS can be applied to a variety of neurologic conditions [16,23,43-48]. Furthermore, these articles noted that TLNS was safe with no reports of serious adverse events [10,16,23,43-47]. Three articles indicated that TLNS would be suitable for use at home [16,43,48].
|
Citation |
Publication Type |
Was CN-NINM described? |
Description of mechanism of action |
Device used |
Indications for use |
Implications for neurorehabilitation |
|---|---|---|---|---|---|---|
|
Lynch P. (2020) |
Literature Review |
Yes |
Neuroplastic changes in the brainstem, pons & cerebellum via activation the cranial nerves V and VII |
Portable Neuromodulation Stimulator (PoNS). |
Stroke, TBI, MS, SCI, CP, Cerebellar Degeneration, Stroke. |
|
|
Diep D, Lam AC, Ko G. (2020) |
Narrative Review |
Yes |
PoNS Induces neuroplastic changes in the brainstem, pons & cerebellum via activation the cranial nerves V and VII |
Portable Neuromodulation Stimulator (PoNS). |
TBI, MS, Stroke & Persons with refractory balance and gait disorders. |
|
|
Danilov Y, Paltin D. (2018) |
Book Chapter |
Yes |
PoNS induces neuroplastic changes in the brainstem, pons & cerebellum via activation the cranial nerves V and VII |
Portable Neuromodulation Stimulator (PoNS). |
TBI |
|
|
Paltin D, Danilov YP, Tyler ME. (2018) |
Book Chapter |
Yes |
PoNS induces neuroplastic changes in the brainstem, pons & cerebellum via activation the cranial nerves V and VII |
Portable Neuromodulation Stimulator (PoNS). |
Stroke |
|
|
Kaczmarek K. (2017) |
Literature Review |
Yes |
Tongue stimulation facilitates neurorehabilitation. No physiological mechanisms reported. |
Portable Neuromodulation Stimulator (PoNS). |
Parkinson’s Disease, SCI, Stroke, Posterior Fossa Syndrome. |
|
|
Danilov et al. (2015) |
Book Chapter |
Yes |
PoNS Induces neuroplastic changes in the brainstem, pons & cerebellum via activation the cranial nerves V and VII. |
Portable Neuromodulation Stimulator (PoNS). |
TBI, MS, Refractory balance and gait disorders. |
|
|
Danilov YP, Kublanov VS. Emerging Noninvasive Neurostimulation Technologies: CN-NINM and SYMPATOCORECTION. JBBS. 2014;04(03):105–13. |
Literature Review |
Yes |
PoNS Induces neuroplastic changes in the brainstem and cerebellum establishing new functional pathways. |
Portable Neuromodulation Stimulator (PoNS) |
Persons with refractory balance. and gait disorders, TBI, MS. |
|
|
Danilov et al. (2014) |
Book Chapter |
Yes |
PoNS Induces neuroplastic changes in the brainstem, pons & cerebellum via activation the cranial nerves V and VII |
Portable Neuromodulation Stimulator (PoNS) |
Refractory balance and gait disorders. |
|
|
Kaczmarek KA. (2011) |
Literature Review |
Yes |
Induced neuroplastic changes via stimulation of the tongue. No physiological mechanisms reported. |
The Tongue Display Unit (TDU) A device created by the author in 1999 to explore electrotactile stimulation on the tongue. |
Persons with refractory balance and gait disorders. |
|
|
TBI: Traumatic Brain Injury; MS: Multiple Sclerosis; SCI: Spinal Cord Injury; CP: Cerebral Palsy |
||||||
Twelve articles were identified from gray literature, 7 of which were research based [14,15,24,26,49-51]. Six articles were research posters [14,15,26,49-51] with one thesis publication (Table 5) [24]. These studies included PD, MS, TBI, and stroke populations. Quality of life was assessed in three studies, one reporting a reduction in depression symptoms [26], one stating a general improvement on the Multiple Sclerosis Impact Scale (MSIS-29) [14] and one reporting improvements in emotion and speech [51]. Three studies found an increase in gait speed [15,26,49] and one study found improved sequencing of muscle activation [49]. Lastly, two studies reported an improvement in static and dynamic eye control [15,50].
|
Citation |
Information Type |
Population |
Intervention |
Device |
Frequency of Intervention |
Outcomes Measures & Intervals |
Results |
Conclusions |
Funding |
|---|---|---|---|---|---|---|---|---|---|
|
Paltin et al. (2017)
|
Conference Poster |
N=1 Chronic stroke |
CN-NINM + Balance, posture, gait, and speech training |
PoNS |
1hr @ 2x/day 30-day withdrawal 1hr @ 2x/day Total of 14 months. |
DIP Assessment: |
DIP increased +59% at day 77, +47% at day 288. QID decreased +75% at day 133, +83% decrease at day 175. SIS: |
CN-NINM can result in long lasting improvements in emotion and speech in patients with chronic stroke. |
University of |
|
Danilov et al. (2013)
|
Conference Poster |
N=1 Stroke |
CN-NINM + flexibility and conditioning |
PoNS Triplets of pulses delivered at 5ms, every 20 ms. |
CN-NINM and daily flexibility, and conditioning exercises delivered 2x/day, 5x week 30-day withdrawal CN-NINM and daily flexibility, and conditioning exercises delivered Total of 13-month intervention
|
DGI Assessment: Pre-Post. |
Improvements seen in: DGI: +48% Significant improvement in static and dynamic eye movement control. |
PoNS is a promising treatment for movement disorders, as well as attention and memory dysfunction. |
University of Wisconsin Foundation. |
|
Danilov et al. (2015)
|
Conference Poster |
N=4 Stroke |
CN-NINM + flexibility and conditioning exercise |
PoNS Triplets of pulses delivered at 5ms, every 20 ms |
2x/day, 5x/week |
Vertical and horizontal gaze. Spontaneous nystagmus. Random saccade and smooth pursuit. Assessment: Pre + Post-test +monthly at home for 12 months. |
Static and dynamic eye control improved. Improved eye fixation and accuracy, smooth pursuit and saccade testing. |
CN-NINM may have benefit for individuals in stroke, PD, MS and TBI as a treatment for oculomotor dysfunction. |
University of Wisconsin Foundation. |
|
Liegle (2013) |
Thesis |
N=1 TBI |
CN-NINM+ target activity |
PoNS |
1 week on/ 1 week off CN-NINM for 5 weeks 20 mins 3x/ day
|
TUG Assessment: baseline, 2,4 & 5 wks |
No significant improvement in TUG, DGI, CBMS, and mGES. Patient reported reduction in tone and confidence |
No significant effects were shown but CN-NINM can potentially provide perceived improvements in tone with minimal risk to the individual. |
Not stated. |
|
Wardini et al. (2017)
|
Conference Poster |
Review of 4 Studies MS, Stroke
|
CN-NINM + balance, gait and breathing awareness training |
PoNS |
Details of the intervention in the 4 studies not provided. |
MSIS-29 |
Statistically significant improvements in balance, gait and QoL. |
PoNS + PT provided improvements in balance, QoL and gait in individuals. |
Not Stated. |
|
Danilov et al. (2012) |
Conference Poster |
N=4 TBI |
CN-NINM + individualized exercise |
PoNS Biphasic waveform with triplet pulses at 5ms intervals, delivered ever 20ms. |
2x/day 5 days/week 2 weeks
|
DGI Gait analysis SOT Assessment: pre-post |
DGI increased (+10-21.5 pts). SOT increased (+10-62pts). Gait analysis: improved muscle sequencing. |
There was a clear improvement in outcomes, however there was a wide variation in improvement between individuals. |
University of |
|
Danilov et al. (2011)
|
Conference Poster |
N=20 PD and MS |
CN-NINM+ flexibility and conditioning |
Placebo CN-NINM + flexibility and conditioning |
2x/ day 5x/week for 2 weeks
|
DGI Assessment: Pre-Post |
Reduced depression, patient reported improvement in MS and PD. symptoms including spasticity, tremor, posture, balance, sleep, facial expression, eye movement, fatigue and bladder control. |
CN-NINM has shown reductions in depression, and patient reported symptoms of MS and PD. |
University of |
|
MS: Multiple Sclerosis; TBI: Traumatic Brain Injury; PD: Parkinson’s Disease; DIP: Dysarthria Impact Profile; QID: Quick Inventory of Depressive Symptoms; SIS: Stroke Impact Scale; DGI: Dynamic Gait Index; SOT: Sensory Organization Test; TUG: Timed Up and GO; RBANS: Repeatable Battery for the Assessment of Neuropsychological Status; CBMS: Community Balance and Mobility Scale; GESM: modified Gait Efficacy Scale; CIQ: Community Integration Questionnaire; MSIS-29: Multiple Sclerosis Impact Scale; ABC: Activity Specific Balance Confidence Scale; CN-NINM: Cranial Nerve Non-Invasive Neuromodulation |
|||||||||
Twelve articles were identified from gray literature, 7 of which were research based [14,15,24,26,49-51]. Six articles were research posters [14,15,26,49-51] with one thesis publication (Table 5) [24]. These studies included PD, MS, TBI, and stroke populations. Quality of life was assessed in three studies, one reporting a reduction in depression symptoms [26], one stating a general improvement on the Multiple Sclerosis Impact Scale (MSIS-29) [14] and one reporting improvements in emotion and speech [51]. Three studies found an increase in gait speed [15,26,49] and one study found improved sequencing of muscle activation [49]. Lastly, two studies reported an improvement in static and dynamic eye control [15,50].
Five of the 12 gray literature articles were conceptual literature articles (Table 6), of which 2 were fact sheets [52,43] and three white papers [54-56]. All five articles reported that the PoNSTM device was used to create neuroplastic change in the brainstem and cerebellum via stimulation of CN V and CN VII, augmenting the effects of rehabilitation to improve balance and gait [52-56]. Two publications indicated TLNS was able to produce long lasting effects [55,56]. The populations discussed included TBI, MS, Cerebral Palsy, stroke, SCI and refractory balance and gait disorders indicating wide applicability for neurological populations. CN-NINM was reported to be safe with no adverse event in two of the publications [55,56] and one reported its applicability for home use [53].
|
Citation |
Publication Type |
Was CN-NINM described? |
Description of mechanism of action |
Device used |
Indications for use |
Implications for neurorehabilitation |
|---|---|---|---|---|---|---|
|
Helius Medical Technologies (2017)
|
Fact sheet |
Yes |
PoNS Induces neuroplastic changes in the brainstem, pons & cerebellum via activation the cranial nerves V and VII. |
Portable Neuromodulation Stimulator (PoNS) |
TBI, MS & CP |
|
|
Helius Medical Technologies (2015) |
Fact sheet |
Yes |
PoNS Induces neuroplastic changes in the brainstem, pons & cerebellum via activation the cranial nerves V and VII. |
Portable Neuromodulation Stimulator (PoNS) |
TBI & MS |
|
|
Backus et al. (2017) |
White Paper |
Yes |
PoNS Induces neuroplastic changes in the brainstem, pons & cerebellum via activation the cranial nerves V and VII. |
Portable Neuromodulation Stimulator (PoNS) |
TBI, Stroke, MS, CP |
|
|
Helius Medical Technologies (2020) |
White Paper |
Yes |
PoNS Induces neuroplastic changes in the brainstem, pons & cerebellum via activation the cranial nerves V and VII. Stimulation induces a tongue stimulation cascade results in neuroplasticity from activity dependent effects on neural connections, impacting motor function, cognition and behavior. |
Portable Neuromodulation Stimulator (PoNS) |
Mild-Moderate TBI, MS, Stroke, Brain Tumor, SCI. |
|
|
Helius Medical Technologies (2019) |
White Paper |
Yes |
PoNS initiates cascades of neural activity. Inducing neuroplastic changes in the brainstem, pons & cerebellum via activation the cranial nerves V and VII. |
Portable Neuromodulation Stimulator (PoNS) |
Refractory balance and gait disorders, Mild-Moderate TBI |
|
|
MS: Multiple Sclerosis; SCI: Spinal Cord Injury; TBI: Traumatic Brain Injury; CP: Cerebral Palsy; CN-NINM: Cranial Nerve Non-Invasive Neuromodulation |
||||||
Discussion
The findings from this scoping review indicate that TLNS combined with physical therapy may enhance neuroplasticity, thereby facilitating neurological recovery in persons with neurological conditions. The majority of papers investigated TLNS as a conjunct modality to be used alongside physiotherapy intervention [8,11,13,15,17,19-22,24-26,35-37,39,41,42,49-51] with relatively few studies investigating the utility of TLNS as an individual treatment modality [9,39]. TLNS via CN-V and CN-VII stimulates multiple subcortical regions leading to modulatory effects impacting clinical outcomes including gait and balance [7,11,16,21,23,36,37,43-45,48]. When paired with rehabilitation interventions, TLNS has been found to improve functional ability [45]. Although these results are encouraging, there is some evidence demonstrating that there is little to no difference in treatment effects between control/sham groups and TLNS groups [21,35,36].
All studies used TLNS via the PoNSTM device, developed at the Tactile Communication Neurorehabilitation Laboratory at the University of Wisconsin-Madison, and licensed by Helius Medical Technologies. The parameters described in the reviewed literature vary widely and are individualized dependent upon the clinical presentation. CN-NINM via TLNS, using devices such as the PoNSTM, are a safe, portable, and feasible modality to include in home-based exercise programs.
Application to clinical populations
CN-NINM via TLNS was investigated in multiple neurological populations including TBI [8,21,24,35,36,41,42,50], MS [14,17,26,37,45], PD [26,45], sub-acute stroke [13-15,51] and spinal cord injury [22]. Other conditions included spinocerebellar ataxia, posterior fossa craniotomy, or were not specified [12,19,20,38-40]. There are limited systematic reviews and randomized controlled studies for all these populations, possibly due to the emergence of TLNS as a relatively new technology. Most studies were non-randomized controlled trials, pilot studies, or small sample research designs. The results of the studies suggest that TLNS can be paired with exercise to improve functional outcomes such as gait and balance across multiple neurological populations.
In Canada, the PoNSTM device is approved for use in persons with mild to moderate TBI and MS and recently achieved FDA approval in the US for MS. The expected number of people living with the effects of CVA, TBI, MS are increasing and are associated with chronic physical and mental disabilities [59]. CN-NINM via TLNS has the potential to improve balance and gait, which are common problems in neurological populations, as well as the potential to influence non-motor impairments such as depression and visual processing thus impacting upon quality of life [14,22,26].
Understanding mechanisms of recovery
Neuroplasticity is a physiological process through which areas of the brain can change or reorganize their functions through intrinsic or extrinsic stimuli [60]. Clinically, this understanding of neuroplasticity forms a theoretical rationale for utilizing modalities such as TLNS [7-12]. CN-NINM via TLNS uses a natural form of stimulation by directly targeting sub-cortical structures via the cranial nerves [18,43]. The anatomical and physiological properties of the tongue allow for localized stimulation through neural impulses or “spikes” stimulating the brainstem and cerebellum [18,43]. Neural networks within the brainstem and cerebellum are key regions involved in postural control, balance and locomotion [61-63]. Recovery of the cortico-reticulospinal system, arising in the brainstem, has been associated with improved gait and lower extremity function [64-66] and is a strong predictor of postural control in adults [67]. The cerebellum, directly connected with the brainstem, coordinates movement, balance, equilibrium, and muscle tone [62] and is associated with motor learning [68].
MRI studies included in this review identified increased activity in the aforementioned areas following application of TLNS [32-35]. TLNS has also shown to increase the volume of grey matter in areas of sub-cortical processing such as the cerebellum and decreases in areas of conscious processing such as the frontal lobe [17]. This indicates a potential recovery mechanism that leads to more typical recovery of functions such as gait and balance that are primarily subserved by sub-cortical neural networks [17]. This neuromodulation is transmitted through the central nervous systems via functionally connected neural systems [48]. CN-NINM via TLNS is therefore quite different from other forms of stimulation, such as transmagnetic stimulation (TMS) and transcranial direct current stimulation (tCDS) which utilize external stimulation through focalized magnetic or electrical fields to induce cortical activity.
A primary goal of neurorehabilitation, and a determinant of quality of life, is achieving independence in walking at home and the community, requiring the integration of postural control and balance [69-71]. Therefore, targeting these neural structures using CN-NINM via TLNS combined with PT has the potential to ameliorate balance and gait impairments [13,35,72].
Dose and safety
A wide range of parameters for pulse-width, frequency, and intensity of the PoNSTM device were used in the literature, however, the optimal parameters remain to be established [7]. For the PoNSTM device, voltage and timing are pre-programmed, however the intensity can be modified through the pulse width and the literature reported ranges from 0.3 to 0.6 µs being used [37,49]. Variable intensities were used in the studies as the device allows for subjects or therapists to alter it to a “comfortable level” [37,49]. Currently, a standardized program has not been established in the literature, however Helius recommends a daily training schedule of two 60-minute sessions and one 20-minute session for six days/week for 14-weeks [54-56]. The application of TLNS was always paired with gait training, balance training, breathing awareness exercises or movement control exercises, however the number and length of sessions per day as well as duration of the intervention period varied [17,19,20,51]. These findings present challenges in determining the optimal parameters for clinical application and a standardized program across studies is necessary to compare intervention effects. No serious adverse events were reported however some side effects included vertigo, pain, headache, vomiting, nausea, and fainting [7,54-56].
Implications for neurorehabilitation
The findings of this scoping review suggest that TLNS primes areas of the CNS for neuroplastic change when combined with PT. The evidence suggests that incorporating motor learning principles such as task specificity and intensity of practice can improve functional outcomes in persons with neurological conditions [73,74]. To maximize neuroplastic change, the activity must be challenging with sufficient intensity to result in an increased production of brain derived neurotrophic factor [75-78]. Therefore, combining CN-NINM via TLNS with neurorehabilitation has the potential to further boost neuroplastic change facilitating functional recovery in activities such as balance and gait [19,20,22,23,48,49] which are common movement problems in people with neurological conditions [4-6]. Furthermore, the wide variety of patient populations studied demonstrates that TLNS has wide applicability to improve balance and gait in persons with neurological conditions. However, it is difficult to ascertain which population, including corresponding clinical indicators, would present as the most amenable candidates to receive this intervention based on the limited sample sizes and relative heterogeneity of populations and participants within current studies.
An additional benefit of TLNS is its safety and portability making it a feasible modality to include in home-based exercise programs. Current neurological rehabilitation models in Canada may not provide sufficient intensity of therapy to maximize functional recovery [79]. The inclusion of TLNS in home-based exercise programs can reduce the economic costs and time-burden of in-person rehabilitation whilst simultaneously increasing accessibility, allowing individuals to further engage in their rehabilitation. Therefore, TLNS has the potential to reduce physical impairments, increasing activity and participation, thereby improving quality of life whilst minimizing health care system costs.
Strengths and Limitations
This is the first scoping review investigating TLNS allowing for exploration of both peer review and gray literature. To date the majority of TLNS literature comprises small sample or non-randomized controlled trials in a wide variety of neurological conditions. Several conceptual papers provide the theoretical underpinning of TLNS and its potential contribution to neuroplastic change in the CNS to optimize recovery of balance and gait in persons with neurological conditions.
Several gaps in the literature on TLNS are identified: (i) determining for whom TLNS is most appropriate including but not limited to the medical diagnosis and clinical impairments most amenable to TLNS; (ii) determining the optimal parameters for use including dose, frequency, intensity and duration of interventions; (iii) determining the specific brain regions in which neuroplastic changes occur; and, (iv) understanding end-user perspectives on TLNS, an area of rehabilitation where research is lacking. Therefore, consideration of mixed-methods studies would provide useful clinical information.
To date, the majority of studies have been undertaken with persons with mild to moderate TBI or MS. There has been limited investigation with persons with stroke despite stroke representing the largest global burden of disability [1,3]. Further research is needed to examine the role of TLNS in this population.
A limitation of a scoping review design is that it does not allow for the assessment of risk of bias in the identified studies [29]. In this review, several studies were completed by researchers with financial interests in the PoNSTM device. Therefore, independent investigation of the effectiveness of TLNS is required. Scoping reviews do not allow for the determination of effectiveness as a statistical analysis cannot be completed [29]. However more robust studies with larger sample sizes are needed before a future systematic review is conducted to determine effect size more accurately. Likewise, the generalizability of the results cannot be determined [29].
Conclusion
With the emergence of new therapeutic technologies, such as the PoNSTM device, for people living with neurological conditions, it is imperative to explore the evidence base for appropriate clinical application and future research directions. The results of this study suggest that TLNS has the potential to produce lasting changes on gait and balance if paired with an individualized physical rehabilitation program. It is a feasible form of treatment that can be incorporated into home exercise programs across various neurological conditions including TBI, MS, CVA, PD and SCI. The existing body of evidence for its application is limited and further research is needed to define parameters for future use.
Disclosures
The authors report no conflict of interest.
International Registered Report Identifier (IRRID): PRR1-10.2196/2996
Financial support: None to declare.
Authorship Statement: Senior researchers JVG and DB conceived the idea of the scoping review and developed the research questions; both contributed meaningfully to drafting and editing the manuscript along with the rest of the research team. TN and SD wrote the introduction. KB developed the study methods. KB, TN, SD, KL, TH, and AB contributed equally to the study selection process and data extraction. KL and SD contributed to the results section. The discussion was written by TH and AB. All authors contributed to and revised the manuscript and approved the final manuscript.
References
2. Thakur KT. Neurological Disorders. Mental, Neurological, and Substance Use Disorders: Disease Control Priorities, 3rd ed. Baltimore , U.S., National Library of Medicine; 2016.
3. Stovner LJ, Hoff JM, Svalheim S, Gilhus, NE. Neurological disorders in the global burden of disease 2010 study. Acta Neurol Scand. 2014;129(s198):1-6.
4. Nonnekes J, Goselink R, Růžička E, Fasano A, Nutt J, Bloem, B. Neurological disorders of gait, balance and posture: a sign-based approach. Nat Rev Neurol. 2018;14(3):183-9.
5. Lai C-H, Chen H-C, Liou T-H, Li W, Chen S-C. Exercise interventions for individuals with neurological disorders: a systematic review of systematic reviews. Am J Phys Med Rehabil. 2019 Oct;98(10):921-30.
6. Coote S, Comber L, Quinn G, Santoyo-Medina C, Kalron A, Gunn H. Falls in people with multiple sclerosis. Int J MS Care. 2020 Nov 1;22(6):247-55.
7. Diep D, Lam ACL, Ko G. A Review of the Evidence and Current Applications of Portable Translingual Neurostimulation Technology. Neuromodulation. 2021 Dec;24(8):1377-1387.
8. Fickling SD, Greene T, Greene D, Frehlick Z, Campbell N, Etheridge T, et al. Brain vital signs detect cognitive improvements during combined physical therapy and neuromodulation in rehabilitation from severe traumatic brain injury: a case report. Front Hum Neurosci. 2020;14:347.
9. Frehlick Z, Lakhani B, Fickling SD, Livingstone AC, Danilov Y, Sackier JM, et al. Human translingual neruostimulation alters resting brain activity in high-density EEG. J. Neuroeng Rehabiloitation. 2019;16(60).
10. Kaczmarek KA.The tongue display unit (TDU) for electrotactile spatiotemporal pattern presentation. Sci Iran D Comput Sci Eng Electr Eng. 2011;18(6):1476-1485.
11. Papa L, LaMee A, Tan CN, Hill-Pryor C. Systematic review and meta-analysis of noninvasive cranial nerve neuromodulation for nervous system disorders. Arch Phys Med Rehabil. 2014;95(12):2435-43.
12. Wildenberg JC, Tyler ME, Danilov YP, Kaczmarek KA, Meyerand ME. Altered connectivity of the balance processing network after tongue stimulation in balance-impaired individuals. Brain Connect. 2013 Feb;3(1):87-97.
13. Galea M, Lizama LEC, Bastani A, Panisset M, Khan F. Cranial nerve non-invasive neuromodulation improves gait and balance in stroke survivors: a pilot randomised controlled trial. Ann Phys and Rehabil Med. 2018;61:e352.
14. Wardini R, Moses M. Portable neuromodulation stimulation (PoNSTM) therapy efficacy for the treatment of traumatic brain injury compared to standard of care. Brain Inj. 2017;31(6-7):806.
15. Danilov YP, Skinner K, Subbotin A, Verbny Y, Tyler M, Kaczmarek K. Effects of CN-NINM intervention on chronic stroke rehabilitation: a case study. In: Society for Neuroscience Annual Meeting 2013.
16. Danilov YP, Tyler ME, Kaczmarek KA, Skinner KL. New approach to neurorehabilitation: cranial nerve noninvasive neuromodulation (CN-NINM) technology. In: Sensing Technologies for Global Health, Military Medicine, and Environmental Monitoring Iv. Bellingham: Spie-Int Soc Optical Engineering; 2014. p. 91120L.
17. Leonard G, Lapierre Y, Chen J-K, Wardini R, Crane J, Ptito A. Noninvasive tongue stimulation combined with intensive cognitive and physical rehabilitation induces neuroplastic changes in patients with multiple sclerosis: a multimodal neuroimaging study. Mult Scler J Exp Transl Clin. 2017;3(1):2055217317690561.
18. Danilov Y, Paltin D. Translingual neurostimulation (TLNS): a novel approach to neurorehabilitation. Phys Med Rehabil. 2017; 4(2).
19. Bastani A., Cofre Lizama LE, Zoghi M, Blashki G, Davis S, Kaye AH, et al. The combined effect of cranial-nerve non-invasive neuromodulation with high-intensity physiotherapy on gait and balance in a patient with cerebellar degeneration: a case report. Cerebellum Ataxias. 2018;5(1):6.
20. Lizama LE, Bastani A, Panisset MG, Drummond K, Khan F, Galea MP. A novel neuromodulation technique for the rehabilitation of balance and gait: A case study. J Clin Neurosci. 2018 Aug;54:140-2.
21. Hou J, Kulkarni A, Tellapragada N, Nair V, Danilov Y, Kaczmarek K, et al. Translingual neural stimulation with the portable neuromodulation stimulator (PoNS®) induces structural changes leading to functional recovery in patients with mild-to-moderate traumatic brain injury. EMJ Radiol. 2020 Sep; 1(1):64-71.
22. Chisholm AE, Malik RN, Blouin J-S, Borisoff J, Forwell S, Lam T. Feasibility of sensory tongue stimulation combined with task-specific therapy in people with spinal cord injury: a case study. J NeuroEng Rehabil. 2014;11:96.
23. Paltin D, Danilov YP, Tyler ME. Brain-machine interfaces: uses and developments. New York: Nova Science Publishers; 2018. Chapter 3, Direct and indirect benefits of translingual neurostimulation technology for neurorehabilitation of chronic stroke symptoms, p. 69-83.
24. Liegl KP. Potential benefits and withdrawal effects of cranial nerve non-invasive neuromodulation on functional mobility for individuals with traumatic brain injury [dissertation]. University of Wisconsin Milwaukee; 2013.
25. Paltin D, Tyler M, Danilov Y. Cognitive enhancement exciting discovery using trans-lingual neuro-stimulation. J Neurol Neurorehabil Res. 2017; 2 (1): 39-45. J Neurol Neurorehabil Res 2017 Volume 2 Issue. 2017;1:13-5.
26. Danilov YP, Tyler ME, Kaczmarek KA. Rehabilitation of multiple sclerosis and Parkinson’s symptoms using cranial nerve non-invasive neuromodulation (CN-NINM). Mov Disord. 2011;26:S3-4.
27. Peters MDJ, Godfrey C, McInerney P, Munn Z, Tricco AC, Khalil, H. Chapter 15: systematic reviews of prevalence and incidence (2020 version). In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis. Adelaide, JBI. 2020 [cited 2020 Sep 4]. Available from https://synthesismanual.jbi.global.
28. Peters MDJ, Godfrey C, McInerney P, Munn Z, Tricco AC, Khalil, H. Chapter 11: Scoping reviews (2020 version). in: Aromataris, E., Munn, Z., editors. JBI Manual for Evidence Synthesis. Adelaide, JBI. 2020 [cited 2020 Sep 4]. Available from https://synthesismanual.jbi.global.
29. Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19-32.
30. Boughen K, Neil T, Dullemond S, Lutowicz K, Bilgasem A, Hastings T, et al. Cranial Nerve Noninvasive Neuromodulation in Adults With Neurological Conditions: Protocol for a Scoping Review. JMIR research protocols. 2021 Jul 28;10(7): e29965.
31. Peters MDJ, Godfrey C, McInerney P, Munn Z, Tricco AC, Khalil, H. Chapter 11: Scoping reviews (2020 version). in: Aromataris, E., Munn, Z., editors. JBI Manual for Evidence Synthesis. Adelaide, JBI. 2020 [cited 2020 Sep 4]. Available from https://synthesismanual.jbi.global.
32. Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Annals of Internal Medicine. 2018 Oct 2;169(7):467-73.
33. Boughen K, Dullemond S, Neil T, Bilgasem A, Lutowicz K, Brooks D, et al. Cranial nerve non-invasive neuromodulation in adults with neurological conditions: a scoping review protocol 2021.
34. Helius Medical Technologies. About Helius [Internet]. 2019. URL: https://heliusmedical.com/index.php/about/overview [accessed 2021-02-18]
35. Ptito A, Papa L, Gregory K, Folmer RL, Walker WC, Prabhakaran V, et al. A prospective, multicenter study to assess the safety and efficacy of translingual neurostimulation plus physical therapy for the treatment of a chronic balance deficit due to mild-to-moderate traumatic brain injury. Neuromodulation: Technology at the Neural Interface. 2021 Dec 1;24(8):1412-21.
36. Tyler M, Skinner K, Prabhakaran V, Kaczmarek K, Danilov Y. Translingual neurostimulation for the treatment of chronic symptoms due to mild-to-moderate traumatic brain injury. Archives of Rehabilitation Research and Clinical Translation. 2019 Dec 1;1(3-4):100026.
37. Tyler ME, Kaczmarek KA, Rust KL, Subbotin AM, Skinner KL, Danilov YP. Non-invasive neuromodulation to improve gait in chronic multiple sclerosis: a randomized double blind controlled pilot trial. Journal of Neuroengineering and Rehabilitation. 2014 Dec;11(1):1-0.
38. Wildenberg JC, Tyler ME, Danilov YP, Kaczmarek KA, Meyerand ME. Electrical tongue stimulation normalizes activity within the motion-sensitive brain network in balance-impaired subjects as revealed by group independent component analysis. Brain Connect. 2011;1(3):255-6
39. Wildenberg JC, Tyler ME, Danilov YP, Kaczmarek KA, Meyerand ME. Sustained cortical and subcortical neuromodulation induced by electrical tongue stimulation. Brain Imaging Behav. 2010;4(3):199-211.
40. Wildenberg JC, Tyler ME, Danilov YP, Kaczmarek KA, Meyerand ME. High-resolution fMRI detects neuromodulation of individual brainstem nuclei by electrical tongue stimulation in balance-impaired individuals. Neuroimage. 2011 Jun 15;56(4):2129-37.
41. Danilov Y, Subbotin A, Skinner K, Verbny Y, Kaczmarek K, Tyler M. Cranial Nerve Non-Invasive Neuromodulation for Symptomatic Treatment of Mild and Moderate Traumatic Brain Injury. J Neurotraum. 2013; A158.
42. D’Arcy RC, Greene T, Greene D, Frehlick Z, Fickling SD, Campbell N, et al. Portable neuromodulation induces neuroplasticity to re-activate motor function recovery from brain injury: a high-density MEG case study. J NeuroEng Rehabil. 2020;17(1):158.
43. Danilov YP, Kublanov VS. Emerging Noninvasive Neurostimulation Technologies: CN-NINM and SYMPATOCORECTION. Journal of Behavioral and Brain Science. 2014; 4: 105-113.
44. Danilov Y, Paltin D. Translingual Neurostimulation (TLNS): Perspective on a Novel Approach to Neurorehabilitation after Brain Injury. Pre-Clinical and Clinical Methods in Brain Trauma Research. 2018; 307-27.
45. Danilov YP, Kublanov VS, Retjunskij KJ, Petrenko TS, Babich MV. Non-invasive Multi-channel Neuro-stimulators in Treatment of the Nervous System Disorders. In: BIODEVICES. 2015 Jan 12; 88-94.
46. Diep D, Lam AC, Ko G. A Review of the Evidence and Current Applications of Portable Translingual Neurostimulation Technology. Neuromodulation: Technology at the Neural Interface. 2021 Dec;24(8):1377-87.
47. Kaczmarek K. The Portable Neurostimulation Stimulator (PoNS) for a neurorehabilitation. Scientia Iranica D. 2015; 24(6). 3171-3180.
48. Lynch P, Roberts D, Monaghan K. Translingual Neurostimulation for Peripheral Motor Control Recovery Post in-Patient Stroke Rehabilitation: A Focus Article. J Alzheimers Neurodegener Dis. 2020;6:038.
49. Danilov Y, Tyler M, Kazcmarek K. New approach to chronic TBI rehabilitation: Cranial nerve noninvasive neuromodulation (CN-NINM technology). J Neurotrauma. 2012;29(10):A97.
50. Danilov Y, Verbny Y, Kaczmarek K, Skinner K, Tyler M. Eye movement rehabilitation by CN-ninm intervention: A set of case studies. J Head Trauma Rehabil. 2015;30(3):E77.
51. Paltin D, Danilov Y, Tyler M. CN-NINM Intervention For The Neurorehabilitation Of Disordered Speech And Emotion. Med Sci Sports Exerc. 2017;49: 31
52. Helius Medical Technologies. The Portable Neuromodulation Stimulator (PoNSTM) Fact Sheet [Internet]. Helius Medical Technologies; 2015 Jun [cited 2021 Jun 25]. Available from: https://heliusmedical.com/images/pdf/Fact_Sheet/2016/PoNS-FACT-SHEET-6-8-2015.pdf
53. Helius Medical Technologies. The Portable Neuromodulation Stimulator (PoNSTM) Fact Sheet [Internet]. Helius Medical Technologies; 2017 Jul [cited 2021 Jun 25]. Available from: https://heliusmedical.com/images/pdf/Fact_Sheet/2017/PoNS-FACT-SHEET_26July2017.pdf
54. Kovelman H. Clinical and economic data supporting consideration of the PoNS for the short-term treatment (14 weeks) of chronic balance deficit due to mild-moderate traumatic brain injury to be used in conjunction with physical therapy. Helius Medical Inc. [2020]
55. Introducing PoNSTM (Portable Neuromodulation Stimulator): PoNS TreatmentTM Restoring balance and gait through noninvasive neuromodulation. Helius Medical Technologies. 2019.
56. Backus D, Hack D, Hauser C, Narayan RK, Rezai A. The Impact of Neuromodulation With the Investigational PoNSTM Therapy on Balance Disorders Due to Traumatic Brain Injury. Helius Technologies. 2017.
57. Danilov YP, Tyler ME, Skinner KL. Efficacy of electrotactile vestibular substitution in patients with bilateral vestibular and central balance loss. Conf Proc IEEE Eng Med Biol Soc. 2006; 6605-9.
58. Danilov YP, Tyler ME, Skinner KL, Hogle RA, Bcaah-y-Rita P. Efficacy of electrotactile vestibular substitution in patients with peripheral and central vestibular loss. J Vestib.Res, 2007;17:119-30.
59. Gaskin J, Gomes J, Darshan S, Krewski D. Burden of neurological conditions in Canada. Neurotoxicology. 2017;61:2-10.
60. Mateos-Aparicio P, Rodríguez-Moreno A. The impact of studying brain plasticity. Front Cell Neurosci. 2019;13:66.
61. Drijkoningen D, Leunissen I, Caeyenberghs K, Hoogkamer W, Sunaert S, Duysens J, et al. Regional volumes in brain stem and cerebellum are associated with postural impairments in young brain-injured patients. Hum Brain Mapp. 2015;36:4897-909.
62. Surgent OJ, Dadalko OI, Pickett KA, Travers BG. Balance and the brain: A review of structural brain correlates of postural balance and balance training in humans. Gait Posture. 2019;71:245-52.
63. Takakusaki K. Functional Neuroanatomy for Posture and Gait Control. J Mov Disord. 2017;10:1-17.
64. Jang SH, Lee SJ. Corticoreticular tract in the human brain: A mini review. Front Neurol. 2019;10:1198.
65. Seo YS, Jang SH. Recovery of gait and injured corticoreticulospinal tracts in a patient with diffuse axonal injury. Neural Regen Res. 2021;16:924.
66. Silva A, Vaughan-Graham J, Silva C, Sousa A, Cunha C, Ferreira R, et al. Stroke rehabilitation and research: consideration of the role of the cortico-reticulospinal system. Somatosens Mot Res; 2018;35:148-52.
67. Boisgontier MP, Cheval B, Chalavi S, van Ruitenbeek P, Leunissen I, Levin O, et al. Individual differences in brainstem and basal ganglia structure predict postural control and balance loss in young and older adults. Neurobiol Aging. 2017;50:47-59.
68. Saywell N, Taylor D. The role of the cerebellum in procedural learning-are there implications for physiotherapists' clinical practice? Physiother Theor Pr. 2008;24:321-328.
69. Schmid AA, Van Puymbroeck M, Altenburger PA, Dierks TA, Miller KK, Damush TM, et al. Balance and Balance Self-Efficacy Are Associated With Activity and Participation After Stroke: A Cross-Sectional Study in People With Chronic Stroke. Arch Phys Med Rehabil. 2012;93:1101-7.
70. Weedersteyn V, de Niet M, van Duijnhoven H, Geurts A. Falls in individuals with stroke. J Rehabil Res Dev. 2008;45:1195-1213.
71. Teasell R, Meyer MJ, Foley N, Salter K. Stroke Rehabilitation in Canada: a work in progress. Top Stroke Rehabil. 2009;16:11-19.
72. Geurts ACH, Haart M De, Nes IJW Van, Duysens J. A review of standing balance recovery from stroke. Gait Posture. 2005;22:267-281.
73. Krakauer JW. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006;19:84-90.
74. Boyd LA, Vidoni ED, Wessel BD. Motor learning after stroke: is skill acquisition a prerequisite for contralesional neuroplastic change?. Neurosci Lett. 2010;482:21-25.
75. Kleim JA, Jones TA. Principles of exercise-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51:225-239.
76. Crozier J, Roig M, Eng JJ, MacKay-Lyons M, Fung J, Ploughman M, et al. High-intensity interval training after stroke: an opportunity to promote functional recovery, cardiovascular health, and neuroplasticity. Neurorehabil Neural Repair. 2018;32:543-56.
77. Mang CS, Snow NJ, Campbell KL, Ross CJD, Boyd LA. A single bout of high-intensity aerobic exercise facilitates response to paired associative stimulation and promotes sequence-specific implicit motor learning. J Appl Physiol. 2014;117:1325-36.
78. Skriver K, Roig M, Lundbye-Jensen J, Pingel J, Helge JO, Kiens B, et al. Acute exercise improves motor memory: exploring potential biomarkers. Neurobiol Learn Mem. 2014;116:46-58.
79. Burridge J, Alt MM, Buurke J, Feys P, Keller T, Klamroth-Marganska V, et al. Neurological rehabilitation: promises and challenges for Canada. Univ Toronto Med J. 2016;93:39-43.