Abstract
Background: Glomerulonephritis refers to a range of conditions involving inflammation and injury to the kidneys' glomeruli, often leading to significant morbidity if left untreated.
Purpose: This review aims to examine emerging advancements in the prevention and treatment of glomerulonephritis and highlight progress in transforming the prognosis of this spectrum of diseases, while also identifying gaps requiring ongoing effort.
Main body: Novel targeted immunotherapies utilizing engineered delivery platforms and biologicals like monoclonal antibodies are progressing in research pipelines, potentially offering safer, more efficacious alternatives to current standard immunosuppression. High-throughput biomarker assays and AI/machine learning algorithms have demonstrated the ability to improve early detection of kidney damage and guide personalized treatment plans. Further prevention opportunities emerge from modulating microbiome-immune interactions, lifestyle factors, and vaccinations shielding against infections triggering renal disorders.
Conclusion: Although challenges remain, recent advancements in unraveling the pathogenesis of glomerulonephritis coupled with the emergence of cutting-edge diagnostics and targeted interventions set the stage for a new era combating the risk and progression of this spectrum of diseases.
Keywords
Glomerulonephritis, Diagnostic tools, Pharmacological treatments, Artificial intelligence, Lifestyle interventions, Nanomedicine
Background
Glomerulonephritis refers to inflammation and injury of the glomeruli, the tiny filtration units within the kidneys. It can arise from various causes like infections, autoimmunity, and genetic factors, and may be acute or chronic [1]. Symptoms include blood and protein in the urine, high blood pressure, and swelling, though manifestations differ. Diagnosis involves exams, lab tests, and imaging studies. Treatment targets the underlying cause, controls symptoms, and prevents complications. Severe disease may require kidney transplant [2]. Glomerulonephritis can lead to chronic kidney disease, end-stage renal disease, and cardiovascular issues, severely impacting health. Thus, effective prevention and management is crucial, through addressing underlying causes, minimizing risks, and adopting healthy lifestyles [3-6]. Recent advances provide new possibilities, including discovering novel biomarkers, imaging techniques, and personalized medicine, plus exploring artificial intelligence, nanotechnology, and stem cell therapy [7].
However, more research, innovation, and patient education are still needed to maximize these opportunities and develop improved prevention, diagnosis, and treatment approaches. Beyond recent advances, prevention strategies like healthy lifestyles, vaccines, and herbal medicine are also being investigated to reduce disease risk. Conventional treatments like immunosuppressants, plasmapheresis, and transplant remain important options. Emerging evidence suggests the gut microbiome may influence development and progression, so modulating it is being explored as a prevention and treatment approach [8]. Other promising research directions are stem cell therapy, telemedicine, remote monitoring, patient support programs, and collaborative data networks.
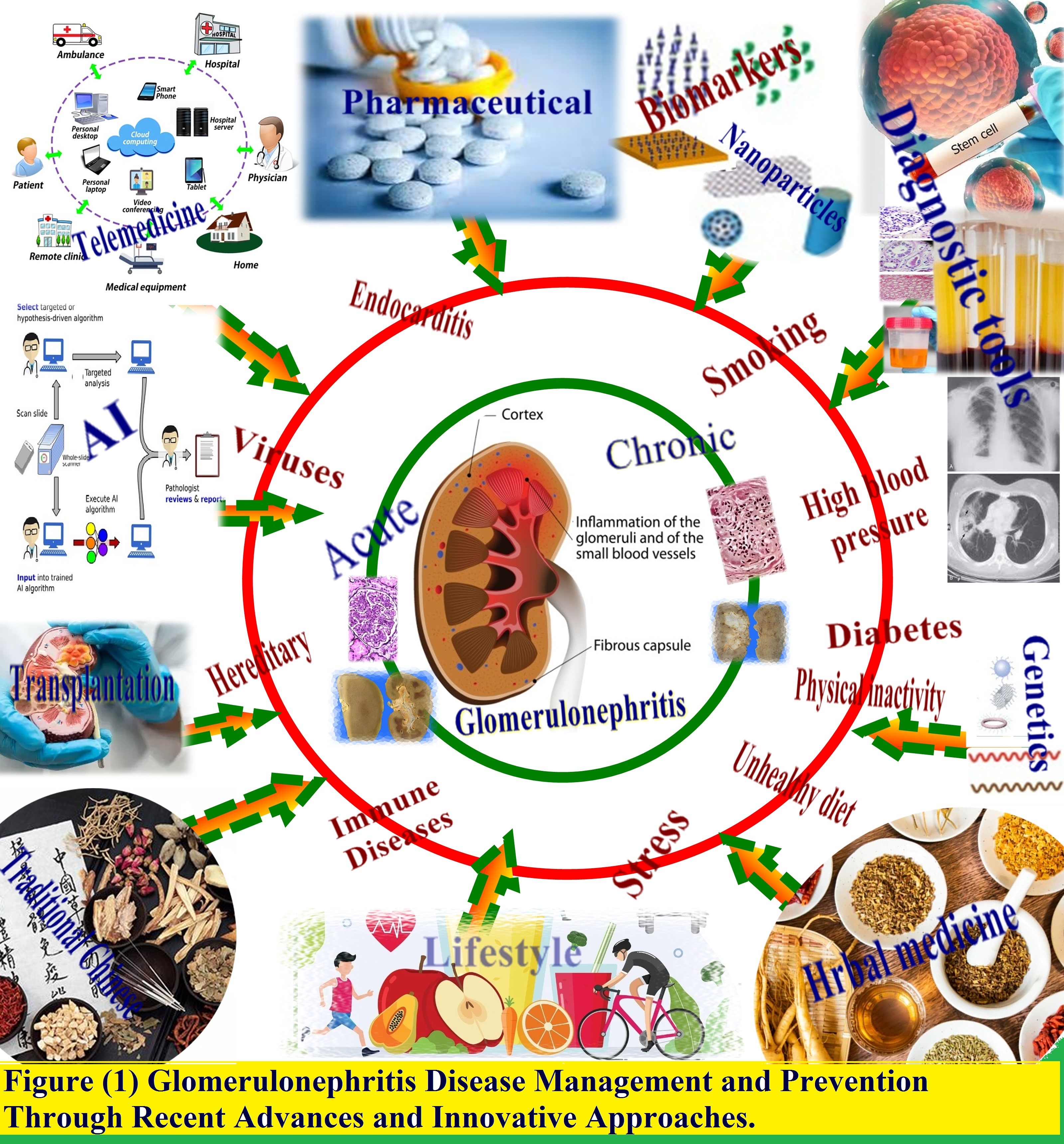
Implementing these advances faces challenges including costs, integration into healthcare systems, ensuring access and efficacy, and sustainability. A collaborative, comprehensive effort among stakeholders is required to overcome these hurdles and realize the goal of improving prevention, diagnosis, and treatment of this disease [9]. As presented in Figure 1, this review examines emerging innovations in combating glomerulonephritis, highlighting progress in comprehending pathogenesis and harnessing tools like nanotechnology and artificial intelligence to pave the pathway to enhanced outcomes.
Figure 1. Glomerulonephritis disease management and prevention through recent advances and innovative approaches.
Prevention of Glomerulonephritis Diseases
Glomerulonephritis, if untreated, can lead to kidney failure. While treatments exist, prevention is the optimal approach. Maintaining a healthy lifestyle, managing underlying conditions, preventing infections, avoiding toxins, and considering herbal medicine are prevention strategies for glomerulonephritis.
Novel diagnostic tools for glomerulonephritis diseases
Glomerulonephritis inflames/damages glomeruli, resulting in symptoms/complications. Early accurate diagnosis is key for treating glomerulonephritis. Recently, novel diagnostic tools emerged including biomarkers, imaging, AI applications, advancing glomerulonephritis understanding as depicted in Table 1. Such innovations potentially enhance diagnostic precision, recognize subtypes, and foresee progression. Additionally, these tools may assist personalized therapy development [10].
|
Technology |
Description |
Applications |
Limitations |
|
AI/ML |
Algorithms analyzing patient data |
Earlier diagnosis; treatment personalization |
Algorithm accuracy; equitability |
|
Biomarkers |
Measurable indicators of disease state |
Detect early kidney injury; indicate subtype |
Lack standardization, specificity |
|
POCT |
Rapid diagnostics at point of care |
Screen for proteinuria, assess kidney function |
Restricted in information gained |
|
Advanced imaging |
Novel scans visualizing kidney changes |
Identify early structural alterations |
Expensive equipment required |
Artificial intelligence and machine learning: Artificial intelligence (AI) and machine learning (ML) are being increasingly explored for their potential in aiding the early detection and diagnosis of glomerulonephritis, a kidney disease that can lead to kidney failure if left untreated. AI and ML algorithms can analyze large amounts of data from various sources, such as electronic health records, imaging studies, and laboratory tests, to identify patterns and make predictions about disease progression. By leveraging these technologies, healthcare providers may be able to detect glomerulonephritis earlier, identify disease subtypes, and develop personalized treatment plans for patients. However, there are challenges to overcome, such as ensuring the accuracy and reliability of AI and ML algorithms, addressing ethical considerations, and ensuring equitable access to these technologies [11].
Biomarkers: Recently, significant progress has been made in the identification and validation of novel biomarkers for the early detection of glomerulonephritis. These biomarkers, which can be detected in blood, urine, or tissue samples, provide insights into disease pathogenesis, identify disease subtypes, and predict disease progression. One example of a protein biomarker is neutrophil gelatinase-associated lipocalin (NGAL), a protein that is upregulated in the setting of kidney injury. NGAL has been shown to be a potential biomarker for the early detection of acute kidney injury and has also been explored as a biomarker for various types of glomerulonephritis, including IgA nephropathy and lupus nephritis. Another example of a biomarker is microRNA-29c, which has been identified as a potential biomarker for diabetic nephropathy, a type of glomerulonephritis associated with diabetes [12]. MicroRNA-29c is involved in the regulation of extracellular matrix proteins, which play a role in the development of diabetic nephropathy. Metabolites such as TMAO and hippurate have also been identified as potential biomarkers for glomerulonephritis. TMAO is produced by gut bacteria and has been implicated in the development of cardiovascular disease and kidney injury. Hippurate, a metabolite produced during the breakdown of amino acids, has been shown to be elevated in patients with IgA nephropathy [13].
Point-of-care testing: Point-of-care testing (POCT) is a convenient and efficient diagnostic method that enables rapid diagnosis and monitoring of diseases at the patient's bedside. In the case of glomerulonephritis, POCT offers an effective and cost-efficient approach for early detection and disease progression monitoring. For instance, the urine dipstick test can detect protein and blood in urine samples, indicating kidney damage and the need for further testing. This readily available and affordable test serves as a practical screening tool for glomerulonephritis. Measuring serum creatinine levels, which estimates glomerular filtration rate (GFR), is another POCT example used to diagnose and monitor glomerulonephritis. Handheld devices enable the rapid and convenient assessment of serum creatinine levels. Additionally, POCT utilizing biomarkers, such as neutrophil gelatinase-associated lipocalin (NGAL), shows promise for the early detection of glomerulonephritis. NGAL testing devices have been developed and demonstrate potential for detecting acute kidney injury and various glomerulonephritis types [14].
Glomerulonephritis imaging: Significant advancements have been made in the development of novel diagnostic imaging tools for early detection of glomerulonephritis. These tools offer non-invasiveness, high sensitivity, and specificity, enabling timely intervention. Contrast-enhanced ultrasound (CEUS) uses microbubble injection to enhance contrast between blood vessels and tissues, providing information on kidney perfusion. CEUS detected changes in kidney perfusion in IgA nephropathy patients before conventional ultrasound [15]. Diffusion-weighted magnetic resonance imaging (DW-MRI) measures water molecule diffusion, revealing microstructural changes in the kidneys. DW-MRI detected early glomerulonephritis changes in IgA nephropathy patients before conventional MRI. Positron emission tomography (PET) and single-photon emission computed tomography (SPECT) are also being explored for early glomerulonephritis detection, utilizing radioactive tracers to assess cellular activity and metabolism in the kidneys [16].
Limitations and challenges of Novel diagnostic tools: Novel diagnostic tools for glomerulonephritis hold promise but have limitations including standardization, specificity, accessibility, required training/expertise, data interpretation complexity, cost, validation requirements, ethical issues, variable patient acceptance, and clinical workflow integration challenges. Standardization is needed for reproducibility, but lack thereof impedes adoption. Many biomarkers lack specificity to accurately diagnose/monitor glomerulonephritis. Some tools like imaging require expensive specialized equipment restricting accessibility. Advanced techniques demand specialized training unavailable in resource-limited settings. Non-experts find artificial intelligence/machine learning results difficult to understand for patient care. Cost prohibits some biomarker assays’ accessibility. Validation establishes accuracy/reliability but takes time and money. Genetic testing raises discrimination concerns if privacy/appropriate care are unaddressed. Lengthy scans/injections receive less acceptance. Providers require training on novel tools’ clinical incorporation [17].
Lifestyle modifications
While medical treatment is essential for managing glomerulonephritis, lifestyle modifications can also play an important role in preventing and managing the disease. Lifestyle modifications may include dietary changes, exercise, smoking cessation, stress management, alcohol consumption, and medication adherence. These modifications can help manage blood pressure, reduce inflammation, prevent further kidney damage, and improve overall health as depicted in Table 2. Adopting a healthy lifestyle can be particularly important for patients with glomerulonephritis, as they are at increased risk of complications such as high blood pressure, cardiovascular disease, and kidney failure. In this way, lifestyle modifications can be an important part of a comprehensive approach to managing and preventing glomerulonephritis. In addition to medical treatment, lifestyle modifications can play an important role in managing glomerulonephritis and improving overall health [18]. Some lifestyle modifications that can be beneficial for patients with glomerulonephritis include:
|
Risk Factor |
Description |
Impact on Disease Risk/Progression |
|
Diet |
High sodium, fat, protein; low potassium, fluids |
Increases inflammation, blood pressure; accelerates kidney damage |
|
Physical inactivity |
Lack of regular exercise |
Worsens CVD risk; reduces kidney function |
|
Smoking |
Cigarette smoking |
Accelerates kidney function decline |
|
Stress |
Chronic psychological stress |
Raises blood pressure, inflammation; damages kidneys |
|
Alcohol |
Excessive intake |
Increases blood pressure, liver toxicity; harmful to kidneys |
|
Medication non-adherence |
Not taking Rx as directed |
Leads to uncontrolled disease, faster progression |
Diet and nutrition: Dietary changes can be an important component of managing glomerulonephritis. A healthy diet may help manage blood pressure, reduce inflammation, and prevent further kidney damage. Patients with glomerulonephritis may benefit from a low-sodium, low-fat, and low-protein diet, along with limiting potassium and phosphorus and staying hydrated. Foods high in sodium, such as processed and canned foods, should be avoided, while healthy fats found in nuts, seeds, and fatty fish can be consumed in moderation. Patients should also aim to consume between 0.6 and 0.8 grams of protein per kilogram of body weight per day and choose high-quality protein sources such as lean meats, fish, and plant-based proteins. In addition to dietary changes, patients with glomerulonephritis should consult with a registered dietitian to develop a personalized nutrition plan that meets their individual needs and preferences [19].
Exercise and physical activity: Regular exercise is an important lifestyle modification for patients with glomerulonephritis. Exercise can help manage blood pressure, improve cardiovascular health, and reduce inflammation, which are all important for managing the disease. Patients with glomerulonephritis should aim to incorporate aerobic exercise, strength training, or low-impact exercises such as yoga or tai chi into their daily routine, as recommended by their healthcare provider. Aerobic exercise, such as brisk walking, cycling, or swimming, can improve cardiovascular health and reduce inflammation. Strength training, such as weightlifting or resistance band exercises, can build muscle mass and improve overall fitness. Low-impact exercises such as yoga or tai chi can reduce stress, improve flexibility, and improve balance [20].
Smoking cessation
For patients with glomerulonephritis, smoking cessation is a crucial lifestyle modification. Smoking can worsen kidney function and increase the risk of cardiovascular disease, which are common complications of glomerulonephritis. Quitting smoking can help improve overall health and reduce the risk of complications associated with the disease. Smoking cessation can improve kidney function, reduce inflammation, lower blood pressure, and decrease the risk of complications such as heart disease, stroke, and kidney failure [21].
Stress management: For patients with glomerulonephritis, stress management is an important aspect of lifestyle modification. Stress can exacerbate kidney function and increase the risk of complications such as high blood pressure and cardiovascular disease. Patients with glomerulonephritis can manage stress by adopting various strategies like mind-body techniques, exercise, social support, and time management. Mind-body techniques such as meditation, yoga, and deep breathing exercises can help reduce stress and promote relaxation. Exercise is an effective stress management technique that can reduce tension and promote relaxation. Social support from family, friends, or support groups can help reduce stress and promote emotional well-being. Effective time management can help reduce stress and improve productivity [22].
Alcohol consumption: Alcohol consumption is a lifestyle factor that patients with glomerulonephritis should manage carefully. Excessive alcohol consumption can worsen kidney function, increase blood pressure, and lead to complications such as liver disease and heart disease [23-29]. Patients with glomerulonephritis should limit alcohol intake to no more than one drink per day for women and two drinks per day for men. They should also avoid binge drinking, which is defined as consuming more than four drinks for women and five drinks for men in a single session. By managing alcohol consumption effectively, patients with glomerulonephritis can reduce the risk of complications associated with the disease. Limiting alcohol intake and avoiding binge drinking can help prevent worsening of kidney function and increase in blood pressure [30].
Medication adherence: Medication adherence is a crucial aspect of managing glomerulonephritis. Patients with glomerulonephritis may require medications to manage blood pressure, reduce inflammation, and prevent complications. Non-adherence to medication regimens can lead to worsening of the disease and an increased risk of complications. Patients with glomerulonephritis can improve medication adherence by understanding the importance of medication, simplifying medication regimens, setting reminders, and engaging in patient education. Healthcare providers can provide support and resources to help patients adhere to their medication regimen effectively [31].
Pharmacological interventions
Pharmacological interventions are a critical component of managing glomerulonephritis. Medications may be used to manage blood pressure, reduce inflammation, and prevent complications. The choice of medication depends on the underlying cause of glomerulonephritis, the severity of the disease, and the patient's medical history. Common medications used to treat glomerulonephritis include Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), immunosuppressants, diuretics, and anticoagulants. These medications work by dilating blood vessels, suppressing the immune system, increasing urine output, and preventing blood clots [32]. Some common medications used to treat glomerulonephritis include:
ACE inhibitors and ARBs: ACE inhibitors and ARBs are commonly used medications to manage blood pressure and reduce proteinuria in patients with glomerulonephritis. These medications work by dilating blood vessels and reducing stress on the kidneys. ACE inhibitors and ARBs are particularly effective in patients with glomerulonephritis who have proteinuria, which is a common complication of the disease. ACE inhibitors and ARBs work by blocking the action of angiotensin II, a hormone that constricts blood vessels and increases blood pressure. By blocking the action of angiotensin II, these medications can dilate blood vessels and reduce blood pressure, which can reduce stress on the kidneys. ACE inhibitors and ARBs can also reduce proteinuria by reducing the amount of protein that is filtered through the kidneys. These medications may also have other beneficial effects, such as reducing inflammation and improving endothelial function [33].
Immunosuppressants: Immunosuppressants are medications that may be used to manage glomerulonephritis by reducing inflammation and preventing damage to the kidneys. Corticosteroids and cytotoxic agents are common immunosuppressants used in the treatment of glomerulonephritis. These medications work by suppressing the immune system, which can reduce inflammation and prevent further damage to the kidneys. Corticosteroids such as prednisone are commonly used to manage glomerulonephritis. They work by reducing inflammation and suppressing the immune response. Cytotoxic agents such as cyclophosphamide and azathioprine may also be used to manage glomerulonephritis. These medications work by inhibiting the growth of cells that contribute to inflammation and damage to the kidneys [34].
Diuretics: Diuretics are medications that may be used to manage fluid retention and reduce blood pressure in patients with glomerulonephritis. Diuretics work by increasing urine output and reducing the amount of fluid in the body. This can help reduce swelling, shortness of breath, and other symptoms associated with fluid retention. Loop diuretics such as furosemide are commonly used in the treatment of glomerulonephritis. These medications work by inhibiting the reabsorption of sodium and chloride in the ascending loop of Henle in the kidneys, which can increase urine output and reduce fluid retention. Thiazide diuretics such as hydrochlorothiazide may also be used in the treatment of glomerulonephritis. These medications work by inhibiting the reabsorption of sodium and chloride in the distal tubules of the kidneys, which can also increase urine output and reduce fluid retention [35].
Anticoagulants: Anticoagulants are medications that may be used to prevent blood clots in patients with glomerulonephritis who are at risk of developing thromboembolic complications. Thromboembolic complications, such as deep vein thrombosis and pulmonary embolism, are a significant risk for patients with glomerulonephritis due to the underlying inflammation and damage to blood vessels. Anticoagulants work by inhibiting the formation of blood clots, which can reduce the risk of thromboembolic complications. Warfarin and heparin are commonly used anticoagulants in the treatment of glomerulonephritis. Warfarin works by inhibiting the production of vitamin K-dependent clotting factors in the liver, which can prevent the formation of blood clots. Heparin works by inhibiting the activity of clotting factors in the blood, which can also prevent the formation of blood clots [36].
Innovative approaches of prevention
The prevention of glomerulonephritis involves controlling risk factors and reducing inflammation to prevent damage to the kidneys. Recent advances in innovative approaches for the prevention of glomerulonephritis include lifestyle modifications, vaccinations, probiotics, anti-inflammatory agents, and genetic screening and counseling. Lifestyle modifications such as regular exercise, maintaining a healthy weight, and quitting smoking have been shown to reduce the risk of developing glomerulonephritis. Vaccinations against infections that can trigger diseases, such as hepatitis B virus (HBV), can also help prevent glomerulonephritis. Probiotics have been suggested to have a role in preventing glomerulonephritis by reducing inflammation and protecting against infections. Anti-inflammatory agents like omega-3 fatty acids, colchicine, and pentoxifylline have shown promise in preventing the development of glomerulonephritis [37].
Genetic testing and risk stratification: Genetic screening constitutes analyzing a person's DNA recognizing changes potentially related to elevated renal disorder or condition probability. Danger stratification identifies higher-hazard individuals reliant on age, sex, ancestral history, and hereditary predisposition. Regarding renal sickness, genetic screening can recognize persons with amplified chance developing selective glomerulonephritis for example Alport syndrome or Fabry disease caused by particular mutations. Early identification enables targeted observation and therapy forestalling or postponing disease starting. Beyond genetic screening, extra chance aspects contain autoimmune or contamination histories, ecologic toxic exposure, and some medicaments [38].
Targeted prevention strategies: Focused risk mitigation techniques aim reducing unique condition likelihood by concentrating on elevated chance persons owing to hereditary, environmental, or lifestyle aspects. Regarding glomerulonephritis, centered deterrents can spot and oversee higher renal disease danger individuals because of particular chance elements. One methodology identifies genetic anomalies raising renal illness probability. Genetic screening determines mutation carriers facilitating targeted supervision and treatment forestalling or postponing disorder debut, as subjects with a relatives’ history of Alport syndrome potentially confronting screening to distinguish related mutations. Other centers controlling causative autoimmune or contamination states contributory to development, for example lupus patients facing individualized immunosuppression to decrease irritation and preclude organ harm [39].
Use of mobile health technology: Mobile health technology, regarded as mHealth, represents a swiftly developing field deploying portable devices like smartphones and tablets delivering medical care and information. Regarding glomerulonephritis prevention, mHealth acts as an instrument promoting healthful behaviors, monitoring risk determinants, and offering education and backing to those vulnerable developing the condition. mHealth can promote prevention by employing apps motivating workouts, nutritional balance, and smoking cessation providing customized recommendations and tracking assistance maintaining health targets. An additional mHealth prevention function involves mobile monitoring of determinants including hemodynamic and glucose employing transmitted data enabling providers targeted intercession for individuals vulnerable developing renal illness. Education and backing may disseminate through texting services and online discussions relaying prevention information and encouragement amid lifestyle changes decreasing condition danger. mHealth technologies may also improve care accessibility for those remote or underserved, possibly confronting barriers accessing traditional providers using telemedicine permitting remote supervision and consultation [40].
Management of Glomerulonephritis Diseases
The management of glomerulonephritis can be classified into acute and chronic forms, each requiring different strategies to manage the disease. Acute glomerulonephritis may involve treating underlying infections or autoimmune disorders that trigger the disease, controlling symptoms such as high blood pressure and excess fluid retention, and preventing complications such as kidney failure. In contrast, chronic glomerulonephritis may involve a combination of medications, lifestyle modifications, and regular monitoring to slow or stop the progression of kidney damage, manage symptoms, and prevent complications such as kidney failure.
Acute management
Acute management of glomerulonephritis involves treating sudden and severe kidney damage and preventing complications. Some of the key strategies for acute management may include:
Immunosuppressive therapy for acute exacerbations: Immunosuppressive therapy constitutes a management approach suppressing the immune system and reducing corporeal inflammation. Regarding glomerulonephritis, immunosuppressive therapy commonly handles exaggerated disease episodes potentially instigating abrupt, grievous renal detriment. During exacerbations, immunological hyperactivation attacks glomeruli, leading to inflammation and harm. Immunosuppressive therapy functions suppressing immunological responses, reducing inflammation, and minimizing additional renal injury. Regularly used immunosuppressants encompass corticosteroids like prednisone and agents consisting of cyclophosphamide and azathioprine, singly or bundled reliant on causation and symptom seriousness. Immunosuppressive therapy's employment amid acute glomerulonephritis exacerbations usually proves effectual in inflammation reduction and subsequent renal harm prevention [41].
Management of nephrotic syndrome: Nephrotic syndrome constitutes a condition typified by amplified urinary protein, diminished serum protein, and edema affecting diverse body regions. Management may couple medications and lifestyle modifications. Medications can include diuretics reducing surplus fluid and edema, ACE inhibitors or ARBs controlling hemodynamic and lessening proteinuria, and immunosuppressants suppressing inflammation. Corticosteroids like prednisone frequently treat nephrotic syndrome causally relating to minimal change disease. Additional immunosuppressants may treat relying on root causation. Lifestyle changes can encompass diminished sodium diet, workout, and smoking cessation. A restricted protein diet may reduce renal workload. Close surveillance of renal performance and proteinuria proves essential treatment assessment and modification [42].
Treatment of acute kidney injury: Acute kidney injury constitutes an abrupt, severe kidney detriment developing from diverse root causes like glomerulonephritis. AKI administration may involve supportive care, for instance dialysis, in addition to managing causative states including contaminations or autoimmune conditions potentially precipitating the occasion. Medications may also regulate hemodynamic and fluid preservation symptoms. Dialysis presents a prevalent AKI treatment particularly when kidneys fail removing waste and surplus fluid. Dialysis employs machinery filtering waste and extra fluid from blood. Principal dialysis varieties encompass hemodialysis utilizing an apparatus externally and peritoneal dialysis exploiting the abdominal lining internally. Beyond dialysis, medications can control hemodynamic, fluid preservation, and electrolyte imbalance symptoms. Reliant on root causation, treatments like antibiotics or immunosuppressants may handle the circumstance. Hospitalization sometimes proves necessary for close supervision and issue management, distinctly for severe AKI threatening dehydration or electrolyte imbalances [43].
Chronic management
Chronic management of glomerulonephritis involves ongoing care and treatment to slow the progression of kidney damage and prevent complications. Some of the key strategies for chronic management may include:
Medical therapy for chronic kidney disease: Medical care for chronic kidney disease aims slowing organ harm progression, symptom management, and complication avoidance. Key strategic methodologies may encompass:
Hemodynamic regulation: Elevated blood pressure commonly arises with CKD and can amplify harm, treated using agents like ACE inhibitors or ARBs lowering pressure and damage prospects [44].
Proteinuria management: Proteinuria also regularly emerges, potentially treated by SGLT-2 inhibitors, ACE inhibitors or ARBs reducing proteinuria and slowing organ deterioration. Anemia mitigation: As anemia commonly develops due to CKD, erythropoietin-stimulating agents may treat fatigue and weakening [45].
Bone disease administration: CKD contributes to low bone mass and fracture probability, treated using supplements like vitamin D and phosphate binders. CKD also imbalances electrolytes including potassium, addressed using diuretics and potassium binders. CKD additionally stems from underlying disorders like diabetes and autoimmunity. Treating causative conditions proves important management [46].
Dialysis and renal replacement therapy: Dialysis and renal replacement therapy constitute management approaches for individuals with progressed chronic kidney disease or renal failure. These techniques aim to replace renal roles through eliminating waste and surplus fluid. Principal dialysis types include hemodialysis employing machinery to filter blood externally while peritoneal dialysis utilizes the abdominal lining for internal blood filtering. Hemodialysis commonly occurs at dialysis facilities or hospitals contrasting peritoneal dialysis's domestic performance. Renal replacement therapy may also involve kidney transplantation substituting the diseased organ with a healthy donor kidney. Transplantation usually follows dialysis inefficacy or tolerance inability [47].
Kidney transplantation: Kidney transplantation constitutes a management approach for individuals with progressed chronic kidney disease or renal failure. This involves replacing the diseased kidney with a healthy donor kidney. Kidney transplantation can improve symptoms, prolong endurance, and progress quality of life for those with renal failure. Donor categories encompass living and deceased [48]. Living donors include relatives, friends, or compatible strangers, while deceased donors consist of individuals consenting to organ donation post-mortem. Prior to transplantation, patients undergo testing to gauge suitability, potentially comprising blood analyses, imaging studies, and mental examinations. Recipients also confront evaluation to pinpoint optimum immunosuppressive treatment preventing transplant rejection. Post-transplantation, lifelong immunosuppressant therapy ensures rejection prevention of the transplanted kidney, as close monitoring of renal performance and drug levels guarantees effective administration [49].
Innovative approaches of management
There are several innovative approaches to the management of glomerulonephritis that are currently being studied. Some of these approaches include:
Precision medicine and personalized treatment: Precision medication and individualized treatment constitute evolving medical methodologies increasingly employed in glomerulonephritis administration. These approaches aim to customize care reliant on one's singular genetic, environmental, and lifestyle factors bettering outcomes and lowering side effect risks. In glomerulonephritis, precision medication involves employing genetic screening and progressive diagnostic tools recognizing root causes in individual patients. By identifying specific genetic anomalies or biomarkers contributory to disease, providers can devise individually-tailored plans targeting causative pathologies. As an example, genetic testing may disclose a mutation amplifying glomerulonephritis risk, allowing customized treatment homing in on this. Personalized glomerulonephritis treatment may necessitate modifying dosage or medication class reliant on age, weight, renal function, and additional factors [50-54]. Providers may adjust immunosuppressant amounts predicated on renal proficiency lessening side effect risks. Individualized treatment may also involve lifestyle changes like nutritional modifications or workouts customized to one's precise needs and preferences [55].
Use of artificial intelligence in diagnosis and treatment: Artificial intelligence represents an innovative healthcare methodology using computational algorithms and machine learning to analyze large data volumes, generating insights bettering diagnosis and care. In glomerulonephritis management, AI may assess patient documentation, identifying patterns assisting providers in more accurate diagnoses and effective treatment planning. AI can aid glomerulonephritis identification by examining patient test results like blood analyses, urine screenings, and imaging studies to identify biomarker patterns associated with the disease [56]. As an example, AI may recognize unique biomarkers in blood or urine correlating with glomerulonephritis, allowing more accurate diagnoses. AI may also facilitate individualized treatment planning for glomerulonephritis patients. Through analyzing patient documentation, AI algorithms can recognize the most effective medications and dosages for particular patients dependent on age, weight, renal function, and additional medical conditions. These assists providers devising treatment optimized for individual needs and circumstances aiming to better outcomes and lessen side effects. Beyond diagnosis and treatment, AI may also advance outcomes by forecasting disease progression and recognizing high-risk complication cases [57].
Advances in regenerative medicine: Regenerative medicine represents an interdisciplinary field aiming to restore or substitute harmed or diseased tissues and organs applying progressive biological and engineering techniques. Recent years have witnessed significant advances in regenerative medicine potentially revolutionizing glomerulonephritis care [58]. A promising regenerative medication approach involves deploying stem cells to repair or regenerate damaged renal tissue. Stem cells constitute undifferentiated cells capable of evolving into discrete cell-types, including kidney cells. Analysts are examining stem cell utilization to mend harmed glomeruli, tiny vasculatures accountable for blood waste product filtering. Early investigations demonstrated promising consequences, suggesting stem cell therapy might constitute an effective glomerulonephritis treatment. An additional promising regenerative medication approach involves tissue engineering's development of artificial kidneys or renal tissue. Tissue engineering applies scaffolds and materials creating three-dimensional structures mimicking natural tissue composition and roles. Analysts are exploring tissue engineering's formation of artificial glomeruli and other renal structures to substitute damaged tissue in glomerulonephritis patients [59].
Future Directions and Challenges
The future of managing glomerulonephritis is promising, with ongoing research and advancements in understanding the disease and potential treatments. There are several future directions and challenges that must be considered in the management of this disease:
Emerging therapies and technologies
Emerging therapies and technologies have the potential to revolutionize the management of glomerulonephritis as depicted in Table 3. Some of the most promising emerging therapies and technologies include:
|
Therapy/Technology |
Description |
Mechanism of Action |
Status/Challenges |
|
Gene therapy |
Introduces genetic material to treat disease |
Corrects genetic mutations driving disease progression |
Early research stage, challenges with delivery and safety |
|
Stem cell therapy |
Uses stem cells to regenerate damaged kidney tissue |
Stem cells differentiate into kidney cells to replace damaged tissue |
Early research, challenges with ensuring cell potency and preventing rejection |
|
Biological therapies (monoclonal antibodies, immunomodulators) |
Drugs targeting immune mediators involved in kidney damage |
Bind complement proteins, cytokines; modulate immune cells |
Some in clinical trials, high costs, side effects |
|
Nanomedicine |
Utilizes nanoscale materials for targeted diagnosis/treatment |
Small size enhances tissue penetration; modular design enables precision delivery |
Early research stage, manufacturing hurdles, toxicity concerns |
Gene therapy: Gene therapy has emerged as a prospective new technique for glomerulonephritis management. Gene therapy involves introducing genetic material into cells to remedy or avert disease. In glomerulonephritis management, gene therapy could rectify genetic mutations contributory to the disease's progression. One possible target for gene therapy in glomerulonephritis management includes the complement system, a protein group implicated in the immune system and kidney damage development in glomerulonephritis. Researchers are exploring employing gene therapy to introduce genes regulating the complement system and preventing renal injury. An additional potential target for gene therapy in glomerulonephritis management involves the podocyte, a specialized renal cell accountable for filtering waste from the blood. Podocyte malfunction can contribute to glomerulonephritis creation. Analysts are investigating using gene therapy to introduce genes promoting podocyte functions and inhibiting kidney injury [60].
Stem cell therapy: Stem cell therapy shows potential as a method for glomerulonephritis management. Stem cells are undifferentiated cells capable of maturing into distinct cell-types, including renal cells. In glomerulonephritis management, stem cell therapy could repair or regenerate harmed kidney tissue. One prospective source of stem cells for glomerulonephritis treatment involves the patient's own bone marrow contents. Bone marrow houses stem cells with the capacity to evolve into renal cells, and analysts are exploring employing these cells to reconstruct damaged glomeruli and other renal structures. Another possible source of stem cells for glomerulonephritis treatment consists of induced pluripotent stem cells. Induced pluripotent stem cells result from reprogramming adult cells into an embryonic stem cell-like condition and can develop into kidney cells. Researchers are investigating using induced pluripotent stem cells to bioengineer artificial glomeruli and other renal structures for replacing damaged tissue in glomerulonephritis cases [61].
Biological therapy: Biological therapy has emerged as a prospective approach for glomerulonephritis management. Biological therapies involve drugs produced from living organisms or their products intended to focus immunologic mechanisms contributory to glomerulonephritis development and progression. Monoclonal antibodies represent one category of biological therapy exhibiting promise for glomerulonephritis management. Monoclonal antibodies comprise laboratory-formulated molecules able to target specific immune proteins. In glomerulonephritis management, monoclonal antibodies can home in on complement system proteins or other immunological elements linked to kidney injury. Immunomodulating agents constitute another classification of biological therapy displaying possibility for glomerulonephritis management. Immunomodulating drugs modify the immune system to avert or handle disease. In glomerulonephritis management, immunomodulating drugs can suppress the immune system and inhibit additional harm to the kidneys [62].
Complement inhibitors: Complement inhibitors hold promise as drugs for managing glomerulonephritis, a condition where the complement system contributes to kidney damage. Eculizumab, a monoclonal antibody targeting C5, has shown potential in reducing kidney damage by inhibiting complement system activation. C1 inhibitor, a protein regulating the complement system, is also being explored as a glomerulonephritis treatment [63].
Nanotechnology: Nanotechnology, utilizing nanoscale materials and devices, has shown promise in the management of glomerulonephritis. Recent advancements include targeted drug delivery and nanosensors for early detection of kidney damage, nanoscale imaging techniques, nanoparticle-based immunotherapy, gene therapy, immunomodulation, and biomaterials for tissue repair [64-66]. These innovations aim to enhance drug efficacy, minimize side effects, detect kidney damage early, provide real-time monitoring, deliver genetic material precisely, modulate immune responses, and regenerate kidney tissue. Nanoscale imaging techniques aid in identifying early signs of kidney damage and monitoring disease progression. However, challenges encompass safety concerns, manufacturing obstacles, regulatory and ethical considerations, targeting efficiency, and immune system clearance [67-71].
Vaccination: Vaccination is a crucial preventive strategy against glomerulonephritis, which can result from infections like Streptococcal infections that trigger immune responses causing kidney damage. Vaccines can prevent such infections and decrease the risk of glomerulonephritis [72]. For instance, the pneumococcal vaccine combats Streptococcus pneumoniae, a common cause of respiratory infections and potential glomerulonephritis inducer. Similarly, the influenza vaccine prevents influenza virus infections that can lead to glomerulonephritis. While vaccination doesn't cure existing kidney damage, it averts further injury and complications. Furthermore, vaccines protect against other infections like hepatitis B virus and human papillomavirus, which can also cause glomerulonephritis. Particularly important for high-risk individuals with chronic kidney disease or diabetes, vaccination is a safe and effective public health measure to reduce glomerulonephritis burden and improve outcomes, despite limitations such as effectiveness, access, hesitancy, booster doses, and inability to reverse kidney damage [73-75].
Probiotics and gut microbiome
The intestinal microbiota plays an important role in regulating the immune system's advancement and modulation [75]. Moreover, disturbances in the intestinal microbiota may contribute to the pathogenesis of glomerulonephritis. Probiotics, which are live microorganisms that can provide benefits to health when adequately consumed, have been proposed as a potential therapy for glomerulonephritis through modulation of both the intestinal microbiota and immune system. Animal and human clinical investigations have demonstrated probiotics' ability to modulate the immune system and decrease the severity of kidney damage in glomerulonephritis. For example, research in rats with glomerulonephritis found treatment with Lactobacillus rhamnosus GG enhanced renal function and reduced inflammation and scarring in the kidneys. Another study in humans with IgA nephropathy, a type of glomerulonephritis, revealed treatment with a probiotic containing Lactobacillus acidophilus, Bifidobacterium bifidum, and Streptococcus thermophilus decreased proteinuria, a key biomarker of kidney injury. Probiotics have exhibited promise for glomerulonephritis management, but limitations and challenges remain, such as restricted understanding of the intestinal microbiota's role in glomerulonephritis pathogenesis and probiotics' mechanisms of action [76].
Herbal and traditional medicine
A growing body of research has identified various herbs and compounds with anti-inflammatory and antioxidant properties that may help reduce kidney damage and inflammation in glomerulonephritis. For example, curcumin and resveratrol, two compounds found in turmeric and grapes respectively, have been shown to reduce inflammation and oxidative stress in the kidneys and may help prevent or manage glomerulonephritis. Traditional Chinese herbal formulas have also been studied for their potential benefits in reducing proteinuria and improving kidney function in glomerulonephritis [77,78]. Flos Abelmoschus manihot and Flos carthami are two herbs commonly used in traditional Chinese medicine that have shown promising results in reducing proteinuria and improving kidney function in patients with glomerulonephritis. Another recent advance in herbal medicine for the management of glomerulonephritis is the use of Centella asiatica, an herb commonly used in Ayurvedic medicine. A study in rats with glomerulonephritis found that a compound derived from Centella asiatica was able to reduce kidney damage and inflammation. However, there are also limitations and challenges to consider, such as limited scientific evidence, lack of standardization, potential for adverse effects, lack of regulation, and inter-individual variability [79,80].
Telemedicine: Telemedicine is an innovative healthcare delivery approach that utilizes technology for remote medical care and consultation. It has gained popularity and has the potential to revolutionize healthcare, especially for patients with glomerulonephritis. Telemedicine offers multiple benefits for managing glomerulonephritis, including improved access to care for patients living in rural or remote areas, increased convenience by eliminating the need for travel, enhanced patient outcomes through increased engagement and adherence, reduced healthcare costs by avoiding hospital stays and emergency visits, and improved provider collaboration for better coordination and quality of care. Despite challenges such as reimbursement and regulatory barriers, telemedicine is expected to address these concerns and become a valuable tool in managing chronic conditions like glomerulonephritis [81].
General challenges in implementing new advances
Implementing advances in glomerulonephritis poses challenges including cost limiting patient access, infrastructure changes needing electronic medical records upgrades, personnel training, protocol adoption, clinical resistance to unfamiliar technologies/therapies, prolonged regulatory approval delaying availability, data privacy/security risk necessitating protection, and variable patient acceptance depending on uncertainties surrounding technologies, treatments, side effects or risks. General barriers involve significant financial investments, healthcare restructuring, overcoming reluctance to transition, ensuring protections amid digital transitions, and addressing unfamiliarity with novelties. Strategies addressing these impediments can help translate scientific innovations into optimized clinical applications benefiting patients [82].
List of Abbreviations
ACE: Angiotensin-Converting Enzyme; AKI: Acute Kidney Injury; AI: Artificial Intelligence; ARBs: Angiotensin Receptor Blockers; CEUS: Contrast-Enhanced Ultrasound; CKD: Chronic Kidney Disease; DW-MRI: Diffusion-Weighted Magnetic Resonance Imaging; GFR: Glomerular Filtration Rate; HBV: Hepatitis B Virus; ML: Machine Learning; mHealth: Mobile Health; NGAL: Neutrophil Gelatinase-Associated Lipocalin; PET: Positron Emission Tomography; POCT: Point-of-Care Testing; SPECT: Single-Photon Emission Computed Tomography; SGLT-2: Sodium-Glucose Co-Transporter 2; TMAO: Trimethylamine-N-oxide
Conclusions
Glomerulonephritis constitutes a complex spectrum of immune-mediated renal disorders instigating significant morbidity. Recent scientific advancements proffer optimism, with innovations in comprehension of immunopathogenesis and microbiome contributions plus progressive treatments like nanomedicines and AI disease modeling exhibiting prospect transforming prevention and care. However, realization of this promise necessitates surmounting hurdles encompassing definitive causation elucidation, diagnostic standardization, therapy optimization balancing efficacy and safety, supportive care delivery model evolution, and addressing associated health disparities. Ultimately comprehending precise disease mechanisms and translating findings into enhanced personalized interventions can accomplish improved outcomes, underscoring the value of ongoing investigation.
Recommendations
Realizing glomerulonephritis’ disease burden reduction mandates a multifaceted approach targeting refined understanding of pathogenesis for directed treatments, early accurate diagnosis, and comprehensive preventative strategies. Attaining these objectives warrants expanded research on genetic and environmental contributions, microbiome, and immune system interplay, deploying progressive technologies like AI and nanomedicine, plus validating and implementing emerging biomarkers, therapies, and multidisciplinary care models. Additionally, beyond novel diagnostic and therapeutic innovations, equally crucial elements include advancing screening protocols, disease education, and lifestyle/dietary modifications for primordial and primary prevention among susceptible populations. Finally ensuring equitable access and affordable delivery of existing and new tools can maximize impact on this disease’s rising incidence. Ultimately a collaborative framework integrating these components across research, policy, public health, and clinical spheres can achieve improved patient quality of life and societal benefits.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
All data is available, and sharing is available as well as publication.
Competing interests
The authors hereby declare that they have no competing interests.
Funding
The corresponding author supplied all study materials. There was no further funding for this study.
Authors' contributions
The authors completed the study protocol and were the primary organizers of data collection and the manuscript's draft and revision process. Tamer A. Addissouky wrote the article and ensured its accuracy. All authors contributed to the discussion, assisted in designing the study and protocol and engaged in critical discussions of the draft manuscript. Lastly, the authors reviewed and confirmed the final version of the manuscript.
Acknowledgements
The authors thank all the researchers, editors, reviewers, and the supporting universities that have made great efforts on their studies. Moreover, we are grateful to the editors, reviewers, and readers of this journal.
References
2. Addissouky TA, Khalil AA, El Agroudy AE. Assessment of potential biomarkers for early detection and management of Glomerulonephritis patients with diabetic diseases. American Journal of Clinical Pathology. 2023;160(Suppl_1):S18-S19.
3. Lim CC, Huang Z, Choo JC. Improving health literacy in disease prevention in glomerulonephritis. International Urology and Nephrology. 2023 Jan;55(1):221-3.
4. Addissouky TA, Megahed FA, Elagroudy AE, El Sayed IE. Efficiency of mixture of olives oil and figs as an antiviral agent: a review and perspective. International Journal of Medical Science and Health Research. 2020 Aug;4(4):107-11.
5. Addissouky TA, El Sayed IE, Ali MM, Wang Y, El Baz A, Elarabany N, et al. Shaping the future of cardiac wellness: exploring revolutionary approaches in disease management and prevention. Journal of Clinical Cardiology. 2024 Jan 5;5(1):6-29.
6. Liu Y, Deng S, Zhang Q, Guo Y, Wang Y, Li T, et al. MLIF modulates microglia polarization in ischemic stroke by targeting eEF1A1. Frontiers in Pharmacology. 2021 Sep 7;12:725268.
7. Addissouky TA, Ali MM, El Sayed IE, Wang Y, El Baz A, Elarabany N, et al. Preclinical promise and clinical challenges for innovative therapies targeting liver fibrogenesis. Archives of Gastroenterology Research. 2023 Nov 14;4(1):14-23.
8. Ameh OI, Ekrikpo U, Bello A, Okpechi I. Current management strategies of chronic kidney disease in resource-limited countries. International Journal of Nephrology and Renovascular Disease. 2020 Oct 12:239-51.
9. Teo WY, Chu SW, Chow LY, Yeam CT, Low LL, Quah JH, et al. Role of Alternative Medical Systems in Adult Chronic Kidney Disease Patients: A Systematic Review of Literature. Cureus. 2022 Dec 23;14(12):32874.
10. Lei R, Vu B, Kourentzi K, Danthanarayana AN, Mohan C, Willson RC. A novel technology for home monitoring of lupus nephritis that tracks the pathogenic urine biomarker ALCAM. Frontiers in Immunology. 2022 Dec 9;13:1044743.
11. Wang Y, Wen Q, Jin L, Chen W. Artificial intelligence-assisted renal pathology: advances and prospects. Journal of Clinical Medicine. 2022 Aug 22;11(16):4918.
12. Addissouky T, Ali M, El Sayed IE, Wang Y. Revolutionary innovations in diabetes research: from biomarkers to genomic medicine. Iranian Journal of Diabetes and Obesity. 2023 Dec 28;15(4):228-42.
13. Anders HJ, Kitching AR, Leung N, Romagnani P. Glomerulonephritis: immunopathogenesis and immunotherapy. Nature Reviews Immunology. 2023 Jul;23(7):453-71.
14. Saddique Z, Faheem M, Habib A, UlHasan I, Mujahid A, Afzal A. Electrochemical Creatinine (Bio) Sensors for Point-of-Care Diagnosis of Renal Malfunction and Chronic Kidney Disorders. Diagnostics. 2023 May 13;13(10):1737.
15. Pruijm M, Aslam I, Milani B, Brito W, Burnier M, Selby NM, et al. Magnetic Resonance Imaging to Diagnose and Predict the Outcome of Diabetic Kidney Disease—Where Do We Stand?. Kidney and Dialysis. 2022 Jul 11;2(3):407-18.
16. Mohammed EH, Kaddourah A, Al Khori N, Djekidel M. The diagnostic value of DMSA scan in differentiating functional pseudo-tumors from malignancies in scarred kidneys: case series and literature review. BMC Nephrology. 2023 May 26;24(1):148.
17. Shushunova NA, Mayorova OA, Prikhozhdenko ES, Goryacheva OA, Kulikov OA, Plastun VO, et al. Targeted Therapy for Glomerulonephritis Using Arterial Delivery of Encapsulated Etanercept. International Journal of Molecular Sciences. 2023 Feb 1;24(3):2784.
18. Kawasoe S, Kubozono T, Salim AA, Yoshimine H, Mawatari S, Ojima S, et al. Development of a risk prediction score and equation for chronic kidney disease: a retrospective cohort study. Scientific Reports. 2023 Mar 27;13(1):5001.
19. Aghwana R, Aiwuyo HO, Ovwasa H, Okoye O, Kweki AG, Unuigbe E. Optimizing Nutrition in Renal Patients: Effects of a Low-Protein Diet Supplemented With Ketoacids. Cureus. 2023 Apr 27;15(4):38205.
20. Weiner DE, Liu CK, Miao S, Fielding R, Katzel LI, Giffuni J, et al. Effect of long-term exercise training on physical performance and cardiorespiratory function in adults with CKD: a randomized controlled trial. American Journal of Kidney Diseases. 2023 Jan 1;81(1):59-66.
21. Eid HA, Moazen EM, Elhussini M, Shoman H, Hassan A, Elsheikh A, et al. The influence of smoking on renal functions among apparently healthy smokers. Journal of Multidisciplinary Healthcare. 2022 Dec 31:2969-78.
22. Hegazy MT, Fayed A, Nuzzolese R, Sota J, Ragab G. Autoinflammatory diseases and the kidney. Immunologic Research. 2023 Aug;71(4):578-87.
23. Addissouky TA, Wang Y, Megahed FA. Novel biomarkers assist in detection of liver fibrosis in HCV patients. Egypt Liver Journal 11: 86.
24. Addissouky TA, El-Agroudy AE, El-Torgoman AMAK, E. El-Sayed IE. Efficacy of Biomarkers in Detecting Fibrosis Levels of Liver Diseases. World Journal of Medical Sciences. 2019;16(1):11-18.
25. Addissouky TA, El Agroudy AE, El-Torgoman AM, El Sayed IE, Ibrahim EM. Efficiency of alternative markers to assess liver fibrosis levels in viral hepatitis B patients. Biomedical Research. 2019 Jan 15;30(2):351-6.
26. Addissouky T. Detecting liver fibrosis by recent reliable biomarkers in viral hepatitis patients. American Journal of Clinical Pathology. 2019 Oct 1;152:S85.
27. El Agroudy AE, Elghareb MS, Addissouky TA, Elshahat EH, Hafez EH. Serum hyaluronic acid as non invasive biomarker to predict liver fibrosis in viral hepatitis patients. Journal of Bioscience and Applied Research. 2016 May 24;2(5):326-33.
28. El Agroudy AE, Elghareb MS, Addissouky TA, Elshahat EH, Hafez EH. Biochemical study of some non invasive markers in liver fibrosis patients. Journal of Bioscience and Applied Research. 2016 May 23;2(5):319-25.
29. Addissouky TA, El Agroudy AE, Khalil AA. Developing a novel non-invasive serum-based diagnostic test for early detection of colorectal cancer. American Journal of Clinical Pathology. 2023 Nov 1;160(Supplement_1):S17.
30. Nayak S, Rehman T, Patel K, Dash P, Alice A, Kanungo S, et al. Factors associated with chronic kidney disease of unknown etiology (CKDu): a systematic review. InHealthcare 2023 Feb 13;11(4):551.
31. Legesse ES, Muhammed OS, Hamza L, Nasir BB, Nedi T. Medication related problems among ambulatory patients with chronic kidney disease at St. Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia. Plos One. 2022 Dec 1;17(12):e0278563.
32. Di Leo V, Annese F, Papadia F, Cara I, Giliberti M, Sallustio F, et al. The Landscape of IgA Nephropathy Treatment Strategy: A Pharmacological Overview. Future Pharmacology. 2023 Jun 8;3(2):517-34.
33. Zeng WL, Yang SK, Song N, Chu FF. The impact of angiotensin converting enzyme insertion/deletion gene polymorphism on diabetic kidney disease: A debatable issue. Nefrologia. 2022 Jul 1;42(4):415-31.
34. Windpessl M, Kostopoulou M, Conway R, Berke I, Bruchfeld A, et al. Preventing infections in immunocompromised patients with kidney diseases: vaccines and antimicrobial prophylaxis. Nephrology Dialysis Transplantation. 2023 Nov;38(Supplement_2):ii40-9.
35. Teles F, Peçanha de Miranda Coelho JA, Albino RM, Verçosa Pacheco FC, Rodrigues de Oliveira E, Silveira MA, et al. Effectiveness of thiazide and thiazide-like diuretics in advanced chronic kidney disease: a systematic review and meta-analysis. Renal Failure. 2023 Dec 31;45(1):2163903.
36. Jacobs R, Verbrugghe W, Dams K, Roelant E, Couttenye MM, Devroey D, et al. Regional citrate anticoagulation in continuous renal replacement therapy: Is metabolic fear the enemy of logic? A systematic review and meta-analysis of randomised controlled trials. Life. 2023 May 17;13(5):1198.
37. Kronbichler A, Bajema I, Geetha D, Säemann M. Novel aspects in the pathophysiology and diagnosis of glomerular diseases. Annals of the Rheumatic Diseases. 2023 May 1;82(5):585-93.
38. Sawaf H, Gudura TT, Dorobisz S, Sandy D, Wang X, Bobart SA. Genetic Susceptibility to Chronic Kidney Disease: Links, Risks and Management. International Journal of Nephrology and Renovascular Disease. 2023 Dec 31:1-5.
39. Alallam B, Choukaife H, Seyam S, Lim V, Alfatama M. Advanced drug delivery systems for renal disorders. Gels. 2023 Feb 1;9(2):115.
40. Khalid H, Khan A, Khan MZ, Mehmood G, Qureshi MS. Machine learning hybrid model for the prediction of chronic kidney disease. Computational Intelligence and Neuroscience. 2023;2023:9266889.
41. Alamilla-Sanchez ME, Alcala-Salgado MA, Alonso-Bello CD, Fonseca-Gonzalez GT. Mechanism of action and efficacy of immunosupressors in lupus nephritis. International Journal of Nephrology and Renovascular Disease. 2021 Dec 11:441-58.
42. Shima H, Doi T, Okamoto T, Inoue T, Tashiro M, Wariishi S, et al. Successful treatment of nephrotic syndrome due to pregnancy-related crescentic IgA nephropathy: a case report. BMC Nephrology. 2023 Apr 10;24(1):92.
43. Turgut F, Awad AS, Abdel-Rahman EM. Acute kidney injury: Medical causes and pathogenesis. Journal of Clinical Medicine. 2023 Jan 3;12(1):375.
44. Lee JG, Joo SJ, Kim SY, Choi JH, Boo KY, Hwang JY, et al. Impact of angiotensin-converting enzyme inhibitors versus angiotensin receptor blockers on clinical outcomes in hypertensive patients with acute myocardial infarction. Plos One. 2023 Mar 9;18(3):e0281460.
45. Kalay Z, Sahin OE, Copur S, Danacı S, Ortiz A, Yau K, et al. SGLT-2 inhibitors in nephrotic-range proteinuria: emerging clinical evidence. Clinical Kidney Journal. 2023 Jan;16(1):52-60.
46. Ku E, Del Vecchio L, Eckardt KU, Haase VH, Johansen KL, Nangaku M, et al. Novel anemia therapies in chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney International. 2023 Oct 1;104(4):655-80.
47. Huijben JA, Kramer A, Kerschbaum J, de Meester J, Collart F, Arévalo OL, et al. Increasing numbers and improved overall survival of patients on kidney replacement therapy over the last decade in Europe: an ERA Registry study. Nephrology Dialysis Transplantation. 2023 Apr;38(4):1027-40.
48. Jankowski E, Schlosser M, Wiech T, Wolf G, Busch M. SARS-CoV-2 infection: a possible trigger for the recurrence of IgA nephropathy after kidney transplantation?. Journal of Nephrology. 2023 Jul;36(6):1683-7.
49. Bai J, Zhang T, Wang Y, Cao J, Duan Z, Ji L, et al. Incidence and risk factors for recurrent focal segmental glomerulosclerosis after kidney transplantation: a meta-analysis. Renal Failure. 2023 Dec 31;45(1):2201341.
50. Addissouky TA, Ali MMA, El Sayed IET, Wang Y, Khalil AA. Translational insights into molecular mechanisms of chemical hepatocarcinogenesis for improved human risk assessment. Advances in Clinical Toxicology. 2024;9(1):294.
51. Addissouky TA, Wang Y, El Tantawy El Sayed I, Majeed MAA, Khalil AA. Emerging technologies and advanced biomarkers for enhanced toxicity prediction and safety pharmacology. Advances in Clinical Toxicology. 2024;9(1):293.
52. Addissouky TA, Wang Y, El Tantawy El Sayed I, Majeed MAA, Khalil AA. Transforming toxicity assessment through microphysiology, bioprinting, and computational modeling. Advances in Clinical Toxicology. 2024;9(1):295.
53. Addissouky TA, El Sayed IE, Ali MM. Regenerating Damaged Joints: The Promise of Tissue Engineering and Nanomedicine in Lupus Arthritis. J Clinical Orthopaedics and Trauma Care. 2024;6(2):083.
54. Addissouky TA, El Sayed IE, Ali MM. Conservative and Emerging Rehabilitative Approaches for Knee Osteoarthritis Management. J Clinical Orthopaedics and Trauma Care. 2024;6(2):082.
55. Addissouky TA, Ali M, Sayed IE, Wang Y. Emerging advanced approaches for diagnosis and inhibition of liver fibrogenesis. The Egyptian Journal of Internal Medicine. 2024 Dec;36(1):19.
56. Krisanapan P, Tangpanithandee S, Thongprayoon C, Pattharanitima P, Cheungpasitporn W. Revolutionizing chronic kidney disease management with machine learning and artificial intelligence. Journal of Clinical Medicine. 2023 Apr 21;12(8):3018.
57. Qin X, Xia L, Zhu C, Hu X, Xiao W, Xie X, et al. Noninvasive evaluation of lupus nephritis activity using a radiomics machine learning model based on ultrasound. Journal of Inflammation Research. 2023 Dec 31:433-41.
58. Wang B, Kim K, Tian M, Kameishi S, Zhuang L, Okano T, et al. Engineered bone marrow stem cell-sheets alleviate renal damage in a rat chronic glomerulonephritis model. International Journal of Molecular Sciences. 2023 Feb 13;24(4):3711.
59. Xu K, Han Y, Huang Y, Wei P, Yin J, Jiang J. The application of 3D bioprinting in urological diseases. Materials Today Bio. 2022 Dec 1;16:100388.
60. Mason WJ, Jafree DJ, Pomeranz G, Kolatsi-Joannou M, Rottner AK, Pacheco S, et al. Systemic gene therapy with thymosin β4 alleviates glomerular injury in mice. Scientific Reports. 2022 Jul 16;12(1):12172.
61. Singh J, Singh S. Review on kidney diseases: types, treatment and potential of stem cell therapy. Renal Replacement Therapy. 2023 Apr 27;9(1):21.
62. Yang Z, Xu H, Gou S, Wu H, Hu Z. Pembrolizumab induced-C3 glomerulonephritis and RBC cast nephropathy: a case report. BMC Nephrology. 2023 May 24;24(1):145.
63. Wooden B, Tarragon B, Navarro-Torres M, Bomback AS. Complement inhibitors for kidney disease. Nephrology Dialysis Transplantation. 2023 Nov;38(Supplement_2):ii29-39.
64. Addissouky TA, Sayed IE, Ali MM, Wang Y, Baz AE, Khalil AA, et al. Latest advances in hepatocellular carcinoma management and prevention through advanced technologies. Egyptian Liver Journal. 2024 Jan 2;14(1):2.
65. Addissouky TA, Wang Y, El Sayed IE, Baz AE, Ali MM, Khalil AA. Recent trends in Helicobacter pylori management: harnessing the power of AI and other advanced approaches. Beni-Suef University Journal of Basic and Applied Sciences. 2023 Sep 2;12(1):80.
66. Addissouky TA, Khalil AA. Detecting lung cancer stages earlier by appropriate markers rather than biopsy and other techniques. American Journal of Clinical Pathology. 2020 Oct;154(Supplement_1):S146-7.
67. Shushunova NA, Mayorova OA, Prikhozhdenko ES, Goryacheva OA, Kulikov OA, Plastun VO, et al. Targeted Therapy for Glomerulonephritis Using Arterial Delivery of Encapsulated Etanercept. International Journal of Molecular Sciences. 2023 Feb 1;24(3):2784.
68. Kylies D, Zimmermann M, Haas F, Schwerk M, Kuehl M, Brehler M, et al. Expansion-enhanced super-resolution radial fluctuations enable nanoscale molecular profiling of pathology specimens. Nature Nanotechnology. 2023 Apr;18(4):336-42.
69. Maali A, Gholizadeh M, Feghhi-Najafabadi S, Noei A, Seyed-Motahari SS, Mansoori S, et al. Nanobodies in cell-mediated immunotherapy: On the road to fight cancer. Frontiers in Immunology. 2023 Jan 25;14:1012841.
70. Sargazi S, Arshad R, Ghamari R, Rahdar A, Bakhshi A, Karkan SF, et al. siRNA‐based nanotherapeutics as emerging modalities for immune‐mediated diseases: A preliminary review. Cell Biology International. 2022 Sep;46(9):1320-44.
71. Jiang B, Zhang Y, Li Y, Chen Y, Sha S, Zhao L, et al. A tissue-tended mycophenolate-modified nanoparticle alleviates systemic lupus erythematosus in MRL/Lpr mouse model mainly by promoting local M2-like macrophagocytes polarization. International Journal of Nanomedicine. 2022;17:3251-67.
72. Addissouky TA, El Sayed IE, Ali MM, Wang Y, El Baz A, Khalil AA, et al. Can vaccines stop cancer before it starts? Assessing the promise of prophylactic immunization against high-risk preneoplastic lesions. Journal of Cellular Immunology. 2023 Nov 29;5(4):127-40.
73. Fabrizi F, Donato MF, Tripodi F, Regalia A, Lampertico P, Castellano G. The Impact of Antiviral Treatment of Hepatitis B Virus after Kidney Transplant and the Latest Insights. Pathogens. 2023 Feb 17;12(2):340.
74. Thammathiwat T, Banjongjit A, Iampenkhae K, Townamchai N, Kanjanabuch T. ANCA associated glomerulonephritis following SARS-CoV-2 vaccination: A case series and systematic review. Vaccines. 2023 May 15;11(5):983.
75. Addissouky TA, Wang Y, El Sayed IE, Khalil AA. Probiotics and Diet Modifications: A Holistic Approach to Tackling Helicobacter pylori with the Help of the Gut Microbiota. PREPRINT (Version 1) available at Research Square, http://dx.doi.org/10.21203/rs.3.rs-3139132/v1
76. Addissouky TA, Khalil AA, El Agroudy AE. Assessing the efficacy of a modified triple drug regimen supplemented with mastic gum in the eradication of helicobacter pylori infection. American Journal of Clinical Pathology. 2023;160(Suppl_1):S19.
77. Addissouky TA, Ali MM, El Sayed IE, Wang Y. Recent advances in diagnosing and treating helicobacter pylori through botanical extracts and advanced technologies. Archives of Pharmacology and Therapeutics. 2023 Nov 3;5(1):53-66.
78. Chen H, Cheng Q. Therapeutic Effects of Jin Shui Bao Capsules on Kidney Diseases. Integrative Medicine in Nephrology and Andrology. 2023 Mar 1;10(1):e00025.
79. Addissouky TA, El Sayed IE, Ali MM, Wang Y, El Baz A, Khalil AA, et al. Molecular Pathways in Sepsis Pathogenesis: Recent Advances and Therapeutic Avenues. Journal of Cellular Immunology. 2024 Jan 20;5(6):174-83.
80. Nayak S, Rehman T, Patel K, Dash P, Alice A, Kanungo S, et al. Factors associated with chronic kidney disease of unknown etiology (CKDu): a systematic review. Healthcare 2023 Feb 13;11(4):551.
81. Odler B, Bruchfeld A, Scott J, Geetha D, Little MA, Jayne DR, et al. Challenges of defining renal response in ANCA-associated vasculitis: call to action?. Clinical Kidney Journal. 2023 Jun;16(6):965-75.