Abstract
Molecular biomarker testing is essential to the work up of metastatic and advanced non-small cell lung carcinoma. Despite molecular testing guidelines proposed by the National Comprehensive Cancer Network, Association for Molecular Pathology, and many others, multiple reports continue to indicate that lung cancer patients are inadequately tested for key molecular biomarkers. Within our hospital system, reflex ordered testing of a panel of molecular biomarkers in all newly diagnosed lung adenocarcinomas was approved and implemented in 2017. Reflex ordered testing demonstrated significant improvements in both turnaround times for reporting of molecular results and frequency of variant detection in these patients. Over time, this reflex ordered testing strategy for molecular biomarkers in lung adenocarcinoma has been maintained and expanded to include additional biomarkers such as NTRK1 and NTRK3 gene rearrangements.
In the current study, we re-assessed the impact of reflex ordered molecular biomarker testing for lung adenocarcinoma in our hospital system two years following its implementation. We evaluated the turnaround times for reporting of reflex ordered molecular biomarker testing, as well as the frequency of variant detection among a cohort of newly diagnosed lung adenocarcinoma specimens sent to our laboratory for molecular testing between September and December 2019. The average turnaround time for reporting of reflex ordered molecular biomarker results in 2019 was comparable to our previous findings, as was the frequency of variant detection. In the current study, the turnaround time for reporting of results for send-out testing of a reflex ordered molecular biomarker took 6 days longer, on average, than the reporting of results for molecular testing performed in-house. Overall, a reflex ordered testing strategy improves turnaround times, improves frequency of variant detection, and standardizes the molecular biomarker work up for lung adenocarcinoma, which may lead to decreased time to initiation of optimal therapy and improved patient outcomes.
Keywords
Non-small cell lung carcinoma, Reflex molecular biomarker testing
Introduction
The National Comprehensive Cancer Network (NCCN) recommends broad molecular biomarker testing in all patients with metastatic non-squamous, non-small cell lung cancer (NSCLC) to identify patients who would benefit from targeted therapy or immunotherapy; at minimum, testing for EGFR mutations, ALK fusions, ROS1 fusions, MET exon 14 skipping mutations, RET rearrangements, and PD-L1 expression levels should be performed [1]. Despite this, reports continue to indicate that patients with NSCLC are not being tested adequately for predictive and prognostic biomarkers prior to the initiation of treatment. A retrospective review of 1,203 lung adenocarcinoma patients reported that the percentage tested for EGFR was 54%, ALK 51%, ROS1 43%, BRAF 29%, RET 17%, MET exon 14 skipping 15%, and ERBB2 11% [2]. Barriers to adequate molecular testing prior to initial treatment in NSCLC may include long turnaround times and non-standardized ordering practices [3].
Our hospital system approved and implemented a reflex ordered molecular biomarker testing strategy for newly diagnosed lung adenocarcinoma in 2017 and observed marked improvement in the average molecular testing turnaround time from 52.6 days in 2016 to 15.6 days in 2018. Following the implementation of standardized reflex ordered molecular biomarker testing strategy, the variant detection rate for these patients also increased from 25.6% in 2016 to 48.8% in 2017 [3]. Since that time, this reflex ordered testing strategy has been maintained within our hospital system and expanded to include additional informative molecular biomarkers for lung adenocarcinoma, such as NTRK1 and NTRK3 gene rearrangements.
The ALKA-372-001, STARTRK-1, and STARTRK-2 clinical trials showed clinically significant response to the tropomyosin receptor kinase inhibitor Entrectinib with acceptable adverse effects in 54% of adult patients with advanced or metastatic NTRK fusion-positive solid tumors. Nineteen percent of the patients enrolled in these trials had NSCLC [4]. In early 2019, NTRK1 and NTRK3 fusions were approved for addition to the reflex ordered molecular biomarker panel for lung adenocarcinoma throughout our hospital system. The current report is an update to our initial findings regarding the clinical utility of reflex ordered molecular biomarker testing for lung adenocarcinoma, with the addition of two molecular biomarkers to the panel. Table 1 details the molecular biomarkers included in our lung adenocarcinoma reflex ordered testing panel, along with the NCCN recommendations for testing in NSCLC and the clinical rationale or therapeutic implication for each biomarker.
Patients and Methods
This study cohort includes all patients (n=95) with newly diagnosed lung adenocarcinoma within our hospital system, whose specimens were received for reflex ordered molecular biomarker testing by the Molecular Diagnostics Laboratory at Houston Methodist Hospital in Houston, Texas from September 2019 to December 2019, regardless of tumor stage. All data was collected with IRB approval. The turnaround times for reporting of in-house performed molecular biomarker test results and reference laboratory performed send-out test results, as well as the rates of variant detection for each gene alteration included in the reflex ordered panel were analyzed. For the purpose of this study, turnaround time is defined as the number of days elapsed between the release of the initial anatomic pathology report and the release of the final molecular pathology report (in-house molecular turnaround time). The send-out turnaround time for MET amplification by fluorescence in situ hybridization (FISH) was also evaluated, and defined as the number of days elapsed between the release of the initial anatomic pathology report and the release of the send-out molecular testing report.
The lung adenocarcinoma reflex ordered molecular biomarker panel approved throughout our hospital system includes (Table 1): EGFR, KRAS, BRAF, ERBB2 gene mutations; ALK, RET, ROS1, NTRK1 and NTRK3 gene rearrangements; MET exon 14 skipping mutations; PD-L1 expression by immunohistochemistry; and MET gene amplification by FISH (send-out). PD-L1 expression by immunohistochemistry was performed and assessed in-house according to standard protocols (SP142 antibody, Ventana). EGFR, KRAS, BRAF, ERBB2 gene mutation analysis was performed in-house by multiplex polymerase chain reaction and single base extension with mass spectrometry analysis (Oncocarta panel and MassArray instrument, Agena Bioscience). Analysis of ALK, RET, ROS, NTRK1 and NTRK3 gene rearrangements and MET exon 14 gene skipping was performed in-house by next generation sequencing of RNA transcripts (FusionPlex, ArcherDx and NextSeq instrument, Illumina). MET gene amplification was performed by FISH at a reference laboratory (NeoGenomics, Fort Meyers, FL). All reflex ordered molecular biomarker testing was performed in-house at the Molecular Diagnostics Laboratory at Houston Methodist Hospital, with the exception of MET gene amplification.
Results
Molecular testing turnaround Time
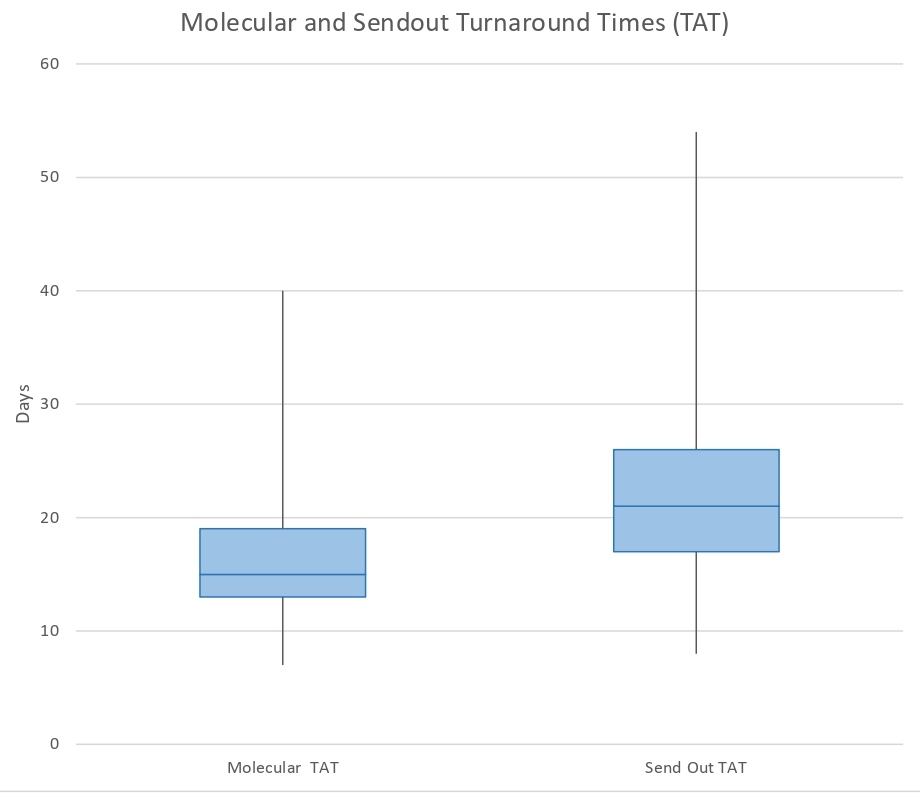
A total of 95 patient specimens with a new histopathologic diagnosis of lung adenocarcinoma of any stage were received for reflex ordered molecular biomarker testing in the Molecular Diagnostics Laboratory at Houston Methodist Hospital from September to December 2019. As shown in Figure 1, the average turnaround time calculated for in-house molecular testing in this patient cohort was 16.6 days (range, 7-40 days; median, 15.0 days). The average turnaround time for MET amplification by FISH send-out testing was 22.6 days (range, 8-54 days; median, 20.5 days) (Figure 1).
Figure 1: Turnaround times for reporting of reflex ordered molecular biomarker testing results for lung adenocarcinoma in 2019. Molecular turnaround times (TAT) for in-house performed molecular biomarker testing at the Houston Methodist Hospital Molecular Diagnostic Laboratory are shown on the left and includes final reporting of results for: EGFR, KRAS, BRAF, ERRB2, and MET exon 14 skipping mutations; ALK, ROS1, RET, NTRK1, NTRK3 gene rearrangements; and PD-L1 expression by immunohistochemistry. As shown in the box and whisker plot, 75% of lung adenocarcinoma patients had in-house performed reflex ordered molecular testing results available within 19 days or less, with an average TAT of 16.6 days and median TAT of 15.0 days (represented by line within box). Send-out TAT for MET amplification testing at a reference laboratory is shown on the right. As shown, 75% of lung adenocarcinoma patients had reflex ordered send-out molecular test results available within 26 days or less, with an average TAT of 22.7 days and median TAT of 21.0 days (represented by line within box).
Variant detection
A variant was identified by reflex ordered molecular biomarker testing 50 of the 95 (52.6%) patient specimens in the current cohort of newly diagnosed lung adenocarcinomas within our hospital system. The variants detected included: 12 EGFR (12.6%) and 29 KRAS (30.5%) mutations; 2 ALK (2.1%) and 2 ROS1 (2.1%) rearrangements; 2 (2.1%) MET exon 14 skipping mutations; and 3 (3.2%) MET amplifications. In this cohort, no mutations of BRAF or ERBB2 or gene rearrangements of RET or NTRK1/3 were identified. PD-L1 tumor cell staining by immunohistochemistry was <50% in 81 of the 95 cases (85.3%) included in the cohort; of these, 62 cases (65%) demonstrated absent PD-L1 staining.
Discussion
Multiple entities, including the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association of Molecular Pathology, recommend molecular biomarker testing for EGFR, ALK, ROS1, and BRAF, in addition to an expanded panel to include gene alterations of KRAS, ERBB2, MET (gene amplification and exon 14 skipping), NTRK, RET and tumor mutational burden when possible in all advanced-stage lung cancers with an adenocarcinoma component [16].
Prior to the implementation of reflex ordered testing for lung adenocarcinoma at Houston Methodist Hospital, the average turnaround time for reporting of molecular biomarker results was 52.6 days using standard ordering practices by clinicians within our hospital system. The average turnaround time for reporting of molecular biomarker results decreased markedly to 15.6 days in 2018, following system-wide approval and adoption of reflex ordered molecular biomarker testing in lung adenocarcinomas [12]. As the current data demonstrates, the in-house turnaround times for reporting of reflex ordered molecular biomarker results has remained stable over time, with an average turnaround time of 16.6 days from September to December 2019. The current cohort also showed higher rates of variant detection (52.6%), compared to the standard ordering practices for molecular biomarker testing in lung adenocarcinomas in 2016 (25.6%). This variant detection rate is comparable to our previous finding of 48.8% following the initial implementation of reflex ordered molecular biomarkers for lung adenocarcinoma within our hospital system in 2017 [3].
The need for send-out testing to a reference laboratory is an important consideration in the adoption of reflex ordered testing within an institution. During our initial implementation of reflex ordered molecular biomarker testing in lung adenocarcinomas within our hospital system, a key component was the clinical validation and insourcing of these tests into the Molecular Diagnostics Laboratory. This contributed, at least in part, to the improvement in turnaround times for reflex ordered molecular biomarker results [3]. In addition, reflex ordered molecular biomarker testing allowed for more streamlined workflows within our laboratory and significantly reduced the amount of tumor tissue required for molecular biomarker testing in often limited lung cancer specimens. In the current study, we wanted to assess the turnaround time for reporting of our single remaining send-out test included in the reflex ordered panel for newly diagnosed lung adenocarcinomas within our hospital system. The average turnaround time for reporting of reflex ordered send-out molecular biomarker testing, specifically MET amplification by FISH, was 22.7 days, as shown in Figure 1. Compared to the reflex ordered molecular biomarker testing performed on the same tissue specimens in our laboratory, reporting of results for the send-out molecular biomarker testing took, on average, 6 days longer.
We have demonstrated that reflex ordered molecular biomarker testing significantly improves turnaround times to results and that this improvement can be maintained over time. However, other factors affecting this metric, which are independent of reflex ordering practices, should not be overlooked. Pre-analytical factors such as inadequate specimen labeling or collection, delays in the delivery of specimens, and inadequate processing or fixation of specimens may all contribute to delays in reporting of molecular test results. Pathologist review and tissue microdissection workflows within the laboratory, as well as nucleic acid extraction procedures should be optimized for efficiency. Management of turnaround times for molecular biomarker testing should also be balanced with cost effectiveness and other considerations. For instance, an increased turnaround time may be acceptable if it allows batching of specimens to avoid excessive wasting of testing reagents [17]. The need for assay troubleshooting and repeat testing may also be an occasional factor in delays for the reporting of molecular test results. Robust quality assurance programs are crucial for identifying recurrent issues in the workflow to improve overall performance and further reduce turnaround times for reflex ordered molecular biomarker testing.
Importantly, the improved turnaround time demonstrated following implementation of a reflex ordered biomarker molecular testing strategy translates into decreased time to optimal treatment for patients with lung adenocarcinoma. A retrospective study of 306 lung adenocarcinoma patients showed an improvement in time to treatment with optimal first-line systemic therapy from 36 days to 24 days with reflex ordered EGFR and ALK molecular testing [14]. The same study also highlighted a significant improvement in the quality of biomarker testing with fewer unsuccessful tests due to inconclusive results, submission of insufficient or inappropriate tissue to testing laboratories, or failure to send tissue to the testing laboratory [18]. Reducing the time to treatment may also lead to decreased mortality for lung cancer patients. A retrospective analysis of multiple solid tumor cancers reported that every week of increased time to treatment was associated with an increased risk of mortality of 3.2% and 1.6% in stage I and II non-small cell lung cancers, respectively [19] (Khorana, et al., 2019). Other reported advantages of reflex ordered molecular testing in lung adenocarcinoma include improved tissue stewardship, increased number of patients tested, decreased costs, and reduced human error [20].
Conclusion
In summary, a reflex ordered testing strategy for molecular biomarkers in lung adenocarcinoma was approved and implemented within our hospital system in 2017. Standardized ordering of a panel of molecular biomarkers by pathologists at the time of diagnosis led to significantly decreased turnaround times for the reporting of critical molecular results for our patients, as well as a higher variant detection rate. As the current study shows, these improvements in turnaround time and the higher variant detection rate have been maintained over time within our hospital system. The addition of NTRK1 and NTRK3 gene rearrangements to the reflex ordered molecular biomarker panel for lung adenocarcinoma did not significantly alter the turnaround time in the current study. This is an important consideration, as additional molecular biomarkers and new targeted therapeutic options for NSCLC continue to emerge. The findings in this study continue to support a standardized, comprehensive strategy for molecular biomarker testing in lung adenocarcinoma. Within our hospital system, the implementation of a reflex ordered molecular testing strategy has significantly increased the number of informative gene alterations detected and ensured the timely delivery of critical molecular results to inform the personalized treatment and management of our patients with lung adenocarcinoma.
References
2. Gierman HJ, Goldfarb S, Labrador M, Weipert CM, Getty B, Skrzypczak SM, Catasus C, Carbral S, Singaraju M, Singleton N, Pai N. Genomic testing and treatment landscape in patients with advanced non-small cell lung cancer (aNSCLC) using real-world data from community oncology practices. Journal of Clinical Oncology. 2019; 37(15).
3. Anand K, Phung T, Bernicker EH, Cagle PT, Olsen RJ, Thomas JS. Clinical Utility of Reflex Ordered Testing for Molecular Biomarkers in Lung Adenocarcinoma. Clin Lung Cancer. 2020 Sep;21(5):437-442.
4. Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. The Lancet Oncology. 2020 Feb 1;21(2):271-82.
5. Keedy VL, Temin S, Somerfield MR, Beasley MB, Johnson DH, McShane LM, et al. American Society of Clinical Oncology provisional clinical opinion: epidermal growth factor receptor (EGFR) mutation testing for patients with advanced non–small-cell lung cancer considering first-line EGFR tyrosine kinase inhibitor therapy. Journal of Clinical Oncology. 2011 May 20;29(15):2121-7.
6. Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. The Lancet Oncology. 2012 Jan 1;13(1): e23-31.
7. Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum–pemetrexed in EGFR T790M–positive lung cancer. New England Journal of Medicine. 2017 Feb 16;376(7):629-40.
8. Soria JC, Tan DS, Chiari R, Wu YL, Paz-Ares L, Wolf J, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. The Lancet. 2017 Mar 4;389(10072):917-29.
9. Kazandjian D, Blumenthal GM, Luo L, He K, Fran I, Lemery S, et al. Benefit-risk summary of crizotinib for the treatment of patients with ROS1 alteration-positive, metastatic non-small cell lung cancer. The Oncologist. 2016 Aug;21(8):974.
10. Odogwu L, Mathieu L, Blumenthal G, Larkins E, Goldberg KB, Griffin N, et al. FDA approval summary: dabrafenib and trametinib for the treatment of metastatic non-small cell lung cancers harboring BRAF V600E mutations. The Oncologist. 2018 Jun;23(6):740.
11. Jackman DM, Miller VA, Cioffredi LA, Yeap BY, Jänne PA, Riely GJ, et al. Impact of epidermal growth factor receptor and KRAS mutations on clinical outcomes in previously untreated non–small cell lung cancer patients: results of an online tumor registry of clinical trials. Clinical Cancer Research. 2009 Aug 15;15(16):5267-73.
12. Chuang JC, Stehr H, Liang Y, Das M, Huang J, Diehn M, et al. ERBB2-mutated metastatic non–small cell lung cancer: response and resistance to targeted therapies. Journal of Thoracic Oncology. 2017 May 1;12(5):833-42.
13. Drilon A, Clark JW, Weiss J, Ou SH, Camidge DR, Solomon BJ, et al. Antitumor activity of crizotinib in lung cancers harboring a MET exon 14 alteration. Nature Medicine. 2020 Jan;26(1):47-51.
14. Drilon A, Rekhtman N, Arcila M, Wang L, Ni A, Albano M, et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm trial. The Lancet Oncology. 2016 Dec 1;17(12):1653-60.
15. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med. 2016 Nov 10;375:1823-33.
16. Lindeman NI, Cagle PT, Aisner DL, Arcila ME, Beasley MB, Bernicker EH, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch Pathol Lab Med. 2018 Mar;142(3):321-346.
17. Cree IA, Deans Z, Ligtenberg MJ, Normanno N, Edsjö A, Rouleau E, et al. Guidance for laboratories performing molecular pathology for cancer patients. Journal of Clinical Pathology. 2014 Nov 1;67(11):923-31.
18. Cheema PK, Menjak IB, Winterton-Perks Z, Raphael S, Cheng SY, Verma S, et al. Impact of reflex EGFR/ALK testing on time to treatment of patients with advanced nonsquamous non–small-cell lung cancer. Journal of Oncology Practice. 2017 Feb;13(2):e130-8.
19. Khorana AA, Tullio K, Elson P, Pennell NA, Grobmyer SR, Kalady MF, et al. Time to initial cancer treatment in the United States and association with survival over time: an observational study. PloS One. 2019 Mar 1;14(3):e0213209.
20. Gregg JP, Li T, Yoneda KY. Molecular testing strategies in non-small cell lung cancer: optimizing the diagnostic journey. Translational Lung Cancer Research. 2019 Jun;8(3):286.