Abstract
SARS-CoV-2 virus infection causes a rare multisystem inflammatory syndrome in children (MIS-C) weeks after the acute infection. Clinicians need to know about a similar syndrome in adults- multisystem inflammatory syndrome in adults (MISC-A). A 38-year-old woman presented MIS-A and was effectively managed with intravenous human immune globulin (IVIG) administration supplemented by hydrocortisone sodium succinate.
Keywords
Multisystem inflammatory syndrome in Children, Multisystem inflammatory syndrome in Adults, MIS-C, MIS-A, Intravenous human immune globulin
Introduction
SARS-CoV-2 virus is the cause of COVID-19 disease. This disease was initially thought to be a pure respiratory illness characterized by cough and fever however it soon became evident that it could cause kidney failure, strokes and involve multiple organ systems as the acute phase evolves into a cytokine storm 10-12 days after symptom onset [1] In April of 2020 reports from Europe [2] and United States highlighted MIS-C which occurs two to four weeks after the initial infection [3]. In response, the Centers for Disease Control and Prevention (CDC) issued a case definition for MIS-C and a case form for reporting and collecting information on this illness in patients below the age of 21 [4]. Individuals older than 21 have been reported to present with a similar illness [5]. The CDC summarized 27 cases involving adults using a working case definition for hospitalized patients with past or recent evidence of SARS-CoV-2 infection who present with evidence of elevated inflammatory markers, absence of pulmonary symptoms, and multisystem involvement [6]. I present the case of a 38-year-old woman who presented with this syndrome.
Case Report
A thirty-eight-year-old Asian woman of Nepalese descent presented to the emergency department (ED) with a two-day history of fever, chills, and body aches. Prior to this, she noted a macular rash on the medial part of both calves and right elbow. 36 days prior she had tested positive for SARS-CoV-2 via a nasopharyngeal swab Polymerase Chain Reaction (PCR) when she presented as an outpatient with a complaint of a week of fever followed by symptom resolution. She had no comorbidities besides a prior cholecystectomy and appendectomy. Vitals showed a temperature: 103.2°F (39.5°C), pulse: 115/minute, a blood pressure: 118/81 mmHg and a respiratory rate of 16/minute. Her weight, height, and body mass index were 63.8 kg, 5 feet (152.4 cm), 27.7 kg/m2 respectively. The SpO2 was 98% on room air. The rest of her examination was unremarkable. Her urinalysis was normal, her C-reactive protein (CRP) was 11.9 mg/dl (0.0- 0.8), her serum creatinine was 0.8 mg/dL. Her liver enzymes were slightly elevated with an aspartate aminotransferase (AST) of 213 IU/L (10-40) and alanine aminotransferase (ALT) of 83 IU/L (6-31). Alkaline Phosphatase was 328 IU/L (40-150) with a slightly elevated total bilirubin of 2.2 mg/dL (0.2-1.2). The white cell count was 11.6 × 109/L and platelets were 218 × 109/L with a hemoglobin of 12.2 g/dL. Sed rate was 46 mm/hr. Lactic acid level was 0.9 mmol/L. Computerized tomographic imaging of the abdomen showed mildly enlarged regional lymph nodes. Influenza test was negative and chest radiograph was normal. After some hydration with her temperature down to 101.4 o F (38.5°C), and she was discharged to follow up with her primary care provider.
She returned to the ED 15 hours later with recurrent fever, leg pain, nausea, vomiting, diarrhea and body aches, lightheadedness, and a headache. The rash was now generalized, pruritic, and tender to palpation. Her vitals now showed a temperature of 103.9°F (39.9°C), pulse of 123/minute and the blood pressure of 96/57 mmHg. She had developed right upper quadrant pain which radiated to the chest. The CRP was now 19.2 mg/dl (0.0- 0.8) and lactic acid was 1.6 mmol/L. Her skin was covered with a blanchable erythematous rash with no induration, vesicles, or bullae (see Figure 1). Examination of her tongue, conjunctiva and vulva did not reveal any mucus membrane involvement. There was no evidence of peripheral lymph node enlargement or splenic enlargement. Her mental status was normal. Sepsis work up was started. Her blood pressure did not respond to 500 ml of Ringers lactate and decreased to 70/49 mmHg. A repeat SARS-CoV-2 test was positive with a Cycle Threshold (CT) value of 42.1 (Cepheid GeneXpert System). She was given 3L of Ringers Lactate and admitted to the intensive care unit (ICU). She was started on norepinephrine and blood cultures were obtained. 200 mg of Intravenous remdesivir was initiated. The patient received only one dose. A lumbar puncture did not show any evidence of meningitis. A day after admission an echocardiogram revealed a left ventricular ejection fraction of 60-65%. Two days later it was down to 35-40% with a normal left ventricular size and wall thickness with sinus bradycardia of 40 beats/minute. Immunoglobulin levels and a connective tissue disease panel were normal. Differential diagnosis included urticaria and urticarial vasculitis with systemic features. Based on her positive SARS-CoV-2 test and the absence of a history of urticaria, a diagnosis of MIS-A was made on her second day of admission and she was started on a hydrocortisone sodium succinate (Solucortef) 50 mg every six hours and a single infusion of human immune globulin (Privigen) infusion of intravenous immune globulin at a dose of 2 g/kg (total of 90 g). Within 24 hours the rash, fever and abdominal pain had resolved, and she did not need norepinephrine. The rest of her hospital stay was focused managing bradycardia, fluids and tapering her steroids. A punch biopsy of the skin of the right calf revealed superficial perivascular lymphohistiocytic inflammatory infiltrate with mild focal vacuolar change.
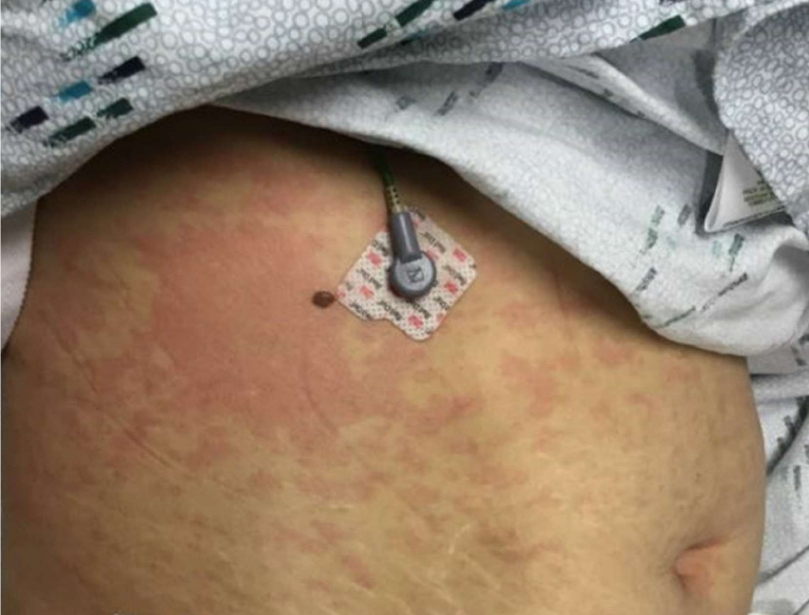
Figure 1: Image of abdominal wall taken on 11/13/2020, the day after admission.
In summary, this is 38-year-old woman who was admitted to the hospital about five weeks after an initial diagnosis of COVID-19 disease with rapidly progressive circulatory shock, fever, and multisystem involvement of the skin, gastrointestinal system, and the cardiovascular system consistent with multisystem inflammatory syndrome in Adults. This was managed with a single dose of intravenous immunoglobulin 2 g/kg and steroids. Within 24 hours the rash had cleared. She was off norepinephrine and was discharged home after nine days in the hospital. The CRP and liver enzymes improved during her hospitalization. Please see Table 1.
|
Lab value (normal levels) |
First ED visit Nov 11 2020 |
Second ED visit Nov 12 2020 |
Nov 14 2020 |
Nov 19 2020 |
|
CRP (0-0.8 mg/dl) |
11.9 |
19.2 |
2.2 |
2.2 |
|
Ferritin (10-200 ng/ml) |
|
|
153 |
130 |
|
Procalcitonin (<0.5ng/dl) |
0.23 |
0.66 |
0.86 |
|
|
D-Dimer (<0.49 µg/ml) |
|
6.52 |
5.66 |
|
|
Sed rate (0-25 mm/hr.) |
46 |
45 |
|
|
|
LDH (51-238 IU/L) |
|
203 |
|
|
|
Il-6 (<1.5 pg/ml) |
|
|
95 |
|
|
WBC (3.2-11 *109/L) |
11.6 |
12.3 |
|
8.0 |
|
Absolute lymphocyte Count (0.8-1.3 *109/L) |
0.6 |
0.2 |
1.4 |
|
|
AST (10-40 IU/L) |
213 |
39 |
27 |
41 |
|
ALT (6-31 IU/L) |
83 |
32 |
24 |
17 |
|
Alkaline phosphatase (40- 150 IU/L) |
328 |
365 |
311 |
299 |
|
Albumin (3.5- 5 g/dL) |
3.5 |
2.1 |
2.0 |
2.0 |
|
BNP (<100 pg/ml) |
|
|
184 |
|
|
Bilirubin (0.2- 1.2 mg/dl) |
2.2 |
2.7 |
1.9 |
0.5 |
Discussion
This patient presented with an acute febrile illness weeks after an acute infection with SARS-CoV-2 infection. Although the cycle threshold value of her initial test was not available the value at her second presentation suggests that at the time of evaluation viral illness was waning. MIS-A and MIS-C both represent post viral syndromes. MIS-A has not been as well defined as its counterpart in children less than 21 years old. MIS-C is a well-defined syndrome comprising of fever, elevated inflammatory markers and multisystem involvement including circulatory shock and often with prominent gastrointestinal involvement. The CDC and various global organizations have formulated a case definition. Because this was initially observed in children and it had many features of Kawasaki disease it was managed as such. However, reports of affected patients being as old as 21 led many authors to distinguish this from Kawasaki disease which primarily manifests in children less than 5 years old. Kawasaki described a postinfectious syndrome coincidentally restricted to children [7]. To date despite large studies not a single microbiologic agent has been found to be the sole cause of Kawasaki disease despite its occurrence in clusters and outbreaks during seasons associated with an increase in respiratory illnesses [8]. MIS-C and MIS-A have opened a fresh door to examine the pathophysiology underlying these postinfectious hyperinflammatory conditions with long term cardiac sequelae. MIS-A and MIS-C may have similar postinfectious immune response pathophysiology just like Kawasaki disease. Age may just be a factor in Kawasaki disease due to an unidentified pathogen which primarily affects children. The common pathway for the multiorgan involvement in MIS-C and Kawasaki disease could be a dysregulated immune response to residual SARS-CoV-2 or the offending pathogen respectively deposited in these tissues.
A possible differential diagnosis in this patient is urticarial vasculitis (UV) which can present with a similar rash with systemic features. Systemic involvement can include the gastrointestinal system, pulmonary, renal, or musculoskeletal system. Although it is mostly idiopathic, it can be associated with autoimmune conditions, drug reactions, infections, and malignancy. This patient had normal levels of Complement C3 at 99 mg/dl (normal 83-177 mg/dl) and C4 at 22 mg/dl (normal15-42 mg/dl) UV is characterized by deposition of antigen antibody complexes in affected vessels. Antinuclear antibodies are usually positive in urticarial vasculitis and was negative in this patient. UV requires the finding of leukocytoclastic vasculitis (LCV) on biopsy. This was absent in this patient. UV also responds to systemic corticosteroids and immunosuppressive agents. UV is a condition that can last for months to years unlike this patient those presentation resolved within 24 hours. The acuity of presentation, the absence of typical laboratory values and rapid resolution makes UV a less likely diagnosis.
The role of intravenous immunoglobulin (IVIG) in Kawasaki disease is well established [9]. Due to the Kawasaki-like features of MIS-C initial cases were managed with (IVIG), steroids, and other immunomodulating agents with remarkable success [10] MIS-A seems to respond equally well to these agents with recovery and a low mortality if recognized early. A common pathophysiology may explain their common response to IVIG. In the absence of a well-defined pathophysiologic model and in view of the multiple proposed but unproven mechanisms by which IVIG works one wonders if we would have used IVIG to manage these cases with such remarkable success given the mystery surrounding COVID-19 disease? I propose we use the term MIS-post COVID or COVID-19 related multisystem inflammatory disease to encompass the presentation in all age groups given all the new knowledge we have at hand. The case definition for children can be duplicated for adults. COVID-19 infection has prominent pulmonary involvement in the setting of active viral infection while MIS- A is a post COVID-19 infection syndrome with minimal to no pulmonary involvement. It is quite rate and is therefore not simply a continuum of COVID-19 disease. The cytokine storm which occurs about 10-12 days after initial symptom onset of COVID-19 disease is characterized by worsening dyspnea, circulatory shock, coagulopathy and elevated inflammatory markers and carries a high mortality. Because of the common presentation with elevated inflammatory markers and hypotension there can easily be confusion between the cytokine storm and MIS-A if the timing in initial positive SARS-CoV-2 test is not considered. The Cycle threshold (CT)value, if available, may be useful in distinguishing acute COVID-19 disease with a low CT value from a post COVID-19 disease with a high CT value as occurred in this patient. The treatments are very different and therefore making the appropriate diagnosis is important. Prior reports have documented residual left ventricular dysfunction as shown in this patient. Treatment guidelines should be formulated based on all these published success stories to include at least a one-time dose of IVIG at 2 g/kg while awaiting more research on this disease. Further research is needed to identify the host factors which predispose some patients to develop this syndrome weeks after the acute illness. Just like patients with Kawasaki disease require follow up for development of coronary artery aneurysms, it may be useful to have guidelines for following these patients up as well.
References
2. Royal College of Paediatrics and Child Health Guidance: Paediatric multisystem inflammatory syndrome temporally associated with COVID-19, https://www.rcpch.ac.uk/sites/default/files/2020-05/COVID-19-Paediatric-multisystem-%20inflammatory%20syndrome-20200501 . Accessed January 10, 2020
3. Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MB, et al. Multisystem inflammatory syndrome in US children and adolescents. New England Journal of Medicine. 2020 Jul 23;383(4):334-46.
4. Centers for Disease Control and Prevention. Emergency preparedness and response: multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19). Health advisory (https://emergency.cdc.gov/han/2020/han00432.asp.
5. Tenforde MW, Morris SB. Multisystem inflammatory syndrome in adults: coming into focus. Chest. 2021 Feb 1;159(2):471-2.
6. Morris SB, Schwartz NG, Patel P, Abbo L, Beauchamps L, Balan S, et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection—United Kingdom and United States, March–August 2020. Morbidity and Mortality Weekly Report. 2020 Oct 9;69(40):1450.
7. Kawasaki T, Kosaki F, Okawa S, Shigematsu I, Yanagawa H. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics. 1974 Sep 1;54(3):271-6.
8. Esper F, Shapiro ED, Weibel C, Ferguson D, Landry ML, Kahn JS. Association between a novel human coronavirus and Kawasaki disease. The Journal of infectious diseases. 2005 Feb 15;191(4):499-502.
9. Burns JC, Franco A. The immunomodulatory effects of intravenous immunoglobulin therapy in Kawasaki disease. Expert review of clinical immunology. 2015 Jul 3;11(7):819-25.
10. Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, et al. Multisystem inflammatory syndrome in children in New York State. New England Journal of Medicine. 2020 Jul 23;383(4):347-58.
