Abstract
In our previous study, we synthesized a novel Fluorinated Trifluoromethyl 4-Thiazolidinone (FTT), which demonstrated promising in vitro anti-cancer activity against Ovarian cancer cells (SKOV3) and Cervical cancer cells (HeLa cells). Hence in the present work, we further investigated it’s probable genotoxic potentials on normal cells in vitro using Chinese Hamster Ovary cells (CHO-K1). Based on the IC50 value in CHO-K1 (4.53 μM), three sub-lethal concentrations were chosen (1, 1.5 and 2 μM) and different cytogenetic toxicity endpoints like induction of chromosome aberrations (CAs), micronuclei (MN) formation, Mitotic index (MI) and Cell cycle studies were performed. The results revealed that, FTT at all the concentrations induced a statistically significant increase in the induction of percentage of aberrant metaphases (P< 0.001, mitotic index (P< 0.001) and induced a lesser percentage of micronuclei compared to vehicle control. A higher percentage of cells in the G1 phase of the cell cycle indicates cell death by FTT occurred may be due to apoptosis mechanism. These preliminary toxicological data establish FTT toxicity profile, providing a fundamental understanding of its safety that is required for further development as a novel anticancer drug.
Keywords
4-Thiazolidinone, CHO-K1, Cytogenetic toxicity
Introduction
Cancer is one of the world's most serious non-communicable diseases, and it has become one of the leading causes of morbidity and mortality. According to the American Cancer Society's projections (2022), approximately 28.0 million new cancer cases and 16.2 million cancer-related deaths are expected to be reported by 2040 [1]. It is imperative to focus more on developing new technologies and pharmacological compounds to treat these cancers. Despite tremendous improvements in cancer prevention and treatment over the past few years, the mortality rate is still too high. Current chemotherapy treatments are frequently constrained by unforeseen adverse effects or developed resistance. Hence, a novelty has become a necessity for cancer research because most existing drugs show not only to cancer cells but also to healthy cells. As a result, patients who receive these drugs frequently experience dreadful side effects on top of occurrence of secondary cancer became very common. As a result, empowerment in research focusing on the development of novel drug molecules or drug candidates that can essentially target and maximize the damage to cancer cells while sparing normal cells and limiting side effects is required.
Many sources are still being investigated, and heterocyclic structures have long received special attention due to their best anti-cancer properties. Thiazolidin-4-one ring system is one of the most intensively studied classes of heterocycles and has been extensively studied in the fields of chemistry and pharmacology. It is represented as a core structure in various synthetic pharmaceuticals, displaying a wide range of biological properties [2]. Earlier in this line of research, several new synthetic 4-Thiazolidinones were successfully synthesised [3] and studied for their anti-cancer properties. The risks and benefits of the FTT must be fully understood, nevertheless, as there is a chance that the advantages of utilising a novel medication as a therapeutic agent surpass the hazards and adverse effects it causes [4]. Previously we have synthesized and reported series of Thiazolidinones-4-One-derivaties, which showed good cytotoxicity activity against cancer cells in which a potent anticancer property was found against Ovarian cancer cells (SKOV3) and Cervical cancer cells (HeLa) [5]. Hence in this study, a comprehensive cytogenetic toxicity assessment of Fluorinated Trifluoromethyl 4-Thiazolidinone (FTT) derivative was performed using normal cells. The resulting data on this genotoxic potential aid in evaluating the risk assessment of FTT, which aids further design and development as a potential cancer chemotherapeutic agent.
Materials and Methods
Compound Name
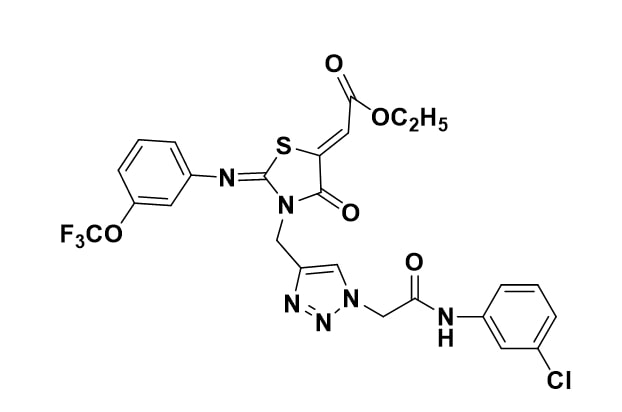
(2Z)-ethyl 2-(3-((1-(2-(3-chlorophenylamino)-2-oxoethyl)-1H-1,2,3-triazol-4-yl)methyl)-4- oxo-2-(3-(trifluoromethyl)phenylimino)thiazolidin-5-ylidene)acetate
Figure 1: Structure.
Spectral data
(2Z)-ethyl 2-(3-((1-(2-(3-chlorophenylamino)-2-oxoethyl)-1H-1,2,3-triazol-4-yl)methyl)-4-
oxo-2-(3-(trifluoromethyl)phenylimino)thiazolidin-5-ylidene)acetate
Yellow solid, mp 167-169°C, IR (KBr) : 3276, 2960, 1690, 1646, 1599, 1324, 1198, 1126 cm-1; 1H NMR (300MHz,CDCl3) δH : 9.13 (1H, s, NH), 8.0 (1H, s, Triazole H), 7.63 (1H, s, ArH), 7.51-7.38 (2H, m, ArH), 7.37-6.99 (5H, m, ArH), 6.89 (1H, s, =CHCO), 5.28 (4H, s, 2NCH2), 4.24 (2H, q, J = 6.6 Hz, OCH2), 1.29 (3H, t, J = 7.0 Hz, OCH2CH3); 13C NMR (300MHz, CDCl3) δc : 165.2, 163.9, 163.3, 162.6, 151.4, 147.2, 141.1, 139.9, 138.8, 133.9, 130.6, 129.6, 128.4, 125.0, 124.0, 123.9, 121.3, 119.4, 117.8, 117.4, 117.0, 61.4, 52.5, 37.3, 13.6; HRMS : m/z calcd for C25H20O4N6ClF3S (M+Na)+ : 615.0799, found 615.0802.
This reported Fluorinated Trifluoromethyl 4-Thiazolidinone (FTT) derivative [5] has been subjected for cytogenetic toxicity studies using normal Chinese hamster ovary cells in vitro.
Cell lines and reagents
Normal Chinese Hamster Ovary Cells (CHO-K1, ATCC® CCL-61™) were obtained from the American Type Culture Collection (ATTC), USA. Cell culture medium, Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were obtained from Sigma-Aldrich, USA.MTT reagents, Propidium iodide was purchased from Sigma-Aldrich, USA.DMSO (Dimethyl sulfoxide), Mitomycin C, acetic acid, methanol, and colchicine were procured from Hi-media, India. All the cell lines were cultured in DMEM supplemented with 10 % FBS and 0.05% antibiotics (penicillin, streptomycin and kanamycin) and maintained in a CO2 incubator at 37°C.
Cell viability assay (MTT assay): The cytotoxicity (MTT assay) of FTT against CHO-K1 cells was evaluated using our previous protocols [6]. CHO-K1 cells were seeded in 96-well plates at a density of 10 × 103 cells per well and grown and incubated with FTT compound at concentrations ranging from 1 to 5 µM for 24 hours. The cells were then incubated for 4 hours with 0.5 mg/mL of MTT reagent, and the resulting insoluble formazan crystals were solubilized with a DMSO: Methanol (1:1 v/v) mixture. The absorbance of the sample in the plate at 570 nm was measured using a Synergy H1 Biotek plate reader. The IC50 value was calculated by plotting concentration vs. percentage cell death.
FACS analysis: The use of an apoptosis-specific fluorochrome, such as PI, that is capable of binding and labeling DNA allows for a rapid and precise evaluation of cellular DNA content and subsequent identification of apoptotic cells. The cell cycle analysis was carried out as per our previous report [6]. To summarize, CHO-K1 cells were treated with 1 m, 1.5 m, and 2 m for 24 hours and incubated in a 5% CO2 incubator. The cells were then collected and fixed overnight in 70% ethanol before being stored at 200C. The cells were washed with 1X PBS and stained for 45 minutes at room temperature with propidium iodide (PI) solution supplemented with RNAse and triton-X. After staining, the cells were once again washed with 1X PBS to remove the unbound PI and then cell cycle analysis was performed using BD Canto FACS, USA.
Cytogenetic toxicity assessment of FTT on CHO-K1 cells in vitro
Based on the cytotoxicity data (MTT test) of CHO-K1 cells, three different sub-lethal concentrations of the FTT (1 µM, 1.5 µM, and 2 µM) were CHO-K1sen and subjected to cytogenetic toxicity studies. All genotoxicity studies were conducted in accordance with OECD guidelines [7].
Chromosome aberration test: Chromosome aberration test was performed to better understand the test compound's clastogenic effects [8]. CHO-K1 cells were treated with three different concentrations of FTT for one cell cycle duration. At 23 hrs of post-treatment, the cells were treated with 0.02% colchicine to arrest the cells in metaphase. Cells were harvested and centrifuged for 5 minutes at 2000 rpm. The supernatant was discarded, and the pellet was centrifuged after being suspended in hypnotic solution (0.9% sodium citrate) for 20-25 minutes at 37°C. The supernatant was discarded, and the cells were fixed in a 3:1 (v/v) methanol/acetic acid solution. The cells were then dropped onto clean, grease free and pre-chilled slides using flame drying method. For each test concentration, at least 100 well-spread metaphase plates were screened for determination of clastogenic effects of the chemical by observing chromatid and chromosomes (gaps and breaks), fragments, minutes, pulverization and translocations. All the tests were repeated thrice.
Mitotic index: Mitotic index study is done to understand the mitotoxic nature of the compound. It provides information on the rate of cell division, in presence of a toxicant which is calculated by the number of dividing cells (Prophase, Metaphase, Anaphase and Telophase) to the total number of cells observed. Same method of chromosome aberration test was followed in preparing the slides for Mitotic index. For this study, at least 2000 cells in triplicates, per each concentration were analyzed to determine the changes in the percentage of mitotic index [9].
Micronucleus test: Micronucleus originates from acentric fragments (chromosome fragments lacking a centromere) or whole chromosomes which are unable to migrate with the rest of the chromosomes during the anaphase of cell division. This test is to detect the formation of these micronuclei in the presence of a test compound. Initially cells were cultured for 24 hrs and then FTT was exposed with three different concentrations (1 µM, 1.5 µM and 2 µM). After the treatment, the cells were washed with PBS and treated with cytochalasin-B (3 μg/ml) for 24 hrs to get bi-nucleated cells. Then the cells were harvested, centrifuged and incubated with 1% Sodium citrate solution for 10 min at 8°C. Later, again centrifuged at 1200 rpm for 5 min and suspended into a fresh hypotonic solution (0.5 ml of Sodium citrate). The pellet was collected and the cells diluted with few drops of Sodium citrate and smeared onto clean slides. The cells were fixed with methanol and stained with 0.5% of Giemsa staining. At least 2000 cells were examined in triplicates to calculate the percentage of MN for each concentration [8].
Results
Using MTT test, cell viability was performed in CHO-K1 cells in order to find out the normal cell toxicity. CHO-K1 cells were treated with FTT in increasing range of concentrations (1 to 5 µM concentrations) and 50% cell death was noticed with 4.53 µM. Based on this cytotoxicity data, three sub-lethal concentrations were CHO-K1sen (1 µM, 1.5 µM and 2 µM) for further cytogenetic studies to estimate the dose dependent toxicity profiling.
Chromosome aberration test
We have investigated the ability of FTT to induce clastogenic effect. It caused chromosome aberrations in a dose-dependent manner at all three tested concentrations. The most common types of aberrations were chromatid and chromosomal gaps and breaks. Cells treated with 1% DMSO as a vehicle control had 26 aberrant metaphases (8.63%) out of 301 metaphases and the total number of chromosomal abnormalities including gaps (9.95%) and excluding gaps (4.9%) were observed. Positive control CHO-K1 cells treated with Mitomycin-C at 2.5 µM exhibited 90 aberrant metaphases (25.63%) out of 351 metaphases, with 56.41% of aberrations including gaps and 40.48% excluding gaps respectively. The chromosome aberrations were mostly with complex chromosome rearrangements like translocations, dicentrics, inversions, tri-radial chromosomes along with chromatid and chromosomal types of aberrations. While comparing the chromosome aberrations, Mitomycin-C induced statistically significant higher number of chromosomes aberrations when compared with DMSO treated cells (P<0.001) (Table 1).
|
Compound |
Dose (µM)
|
No. of metaphases observed |
No. of aberrant metaphases |
Types of aberrations |
% of aberrant metaphase |
Total no. of aberrations |
% of aberrations including gaps
|
% of aberrations excluding gaps |
||||||||
|
Chromatid type of aberrations
|
Chromosome type of aberrations |
F |
M |
Tr |
Qr |
Di |
||||||||||
|
G |
B |
G |
B |
|||||||||||||
|
DMSO (Vehicle control) |
1% |
301 |
26 |
15 |
13 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
8.63 ± 0.85 |
30 |
9.95 ± 1.69 |
4.96 ± 0.96 |
|
FTT |
1 |
315 |
56 |
42 |
30 |
3 |
0 |
0 |
1 |
0 |
0 |
0 |
17.88 ± 1.25*** |
80 |
25.39 ± 3.23 * |
10.15 ± 1.73 (ns) |
|
1.5 |
307 |
61 |
38 |
48 |
3 |
1 |
1 |
1 |
0 |
0 |
0 |
19.89 ± 0.66*** |
97 |
31.59 ± 4.22** |
17.26± 3.16* |
|
|
2 |
302 |
62 |
31 |
45 |
4 |
0 |
3 |
3 |
0 |
1 |
0 |
20.52 ± 0.86*** |
97 |
32.11 ± 0.87 ** |
19.20± 1.31** |
|
|
Mitomycin-C (Positive control) |
2.5 |
351 |
90 |
34 |
46 |
11 |
5 |
4 |
7 |
9 |
0 |
25 |
25.63 ± 0.23*** |
198 |
56.41± 3.53*** |
40.48 ± 3.03*** |
|
±: Standard error of mean; G: Gap; B: Break; F: Fragment; M: Minute; Tr: Triradial; Qr: Quadriradial; Dic: Dicentric; ***P<0.001, **P<0.01, *P<0.05 significant level compared to vehicle control (1% DMSO using Dunnett's Multiple Comparison Test |
||||||||||||||||
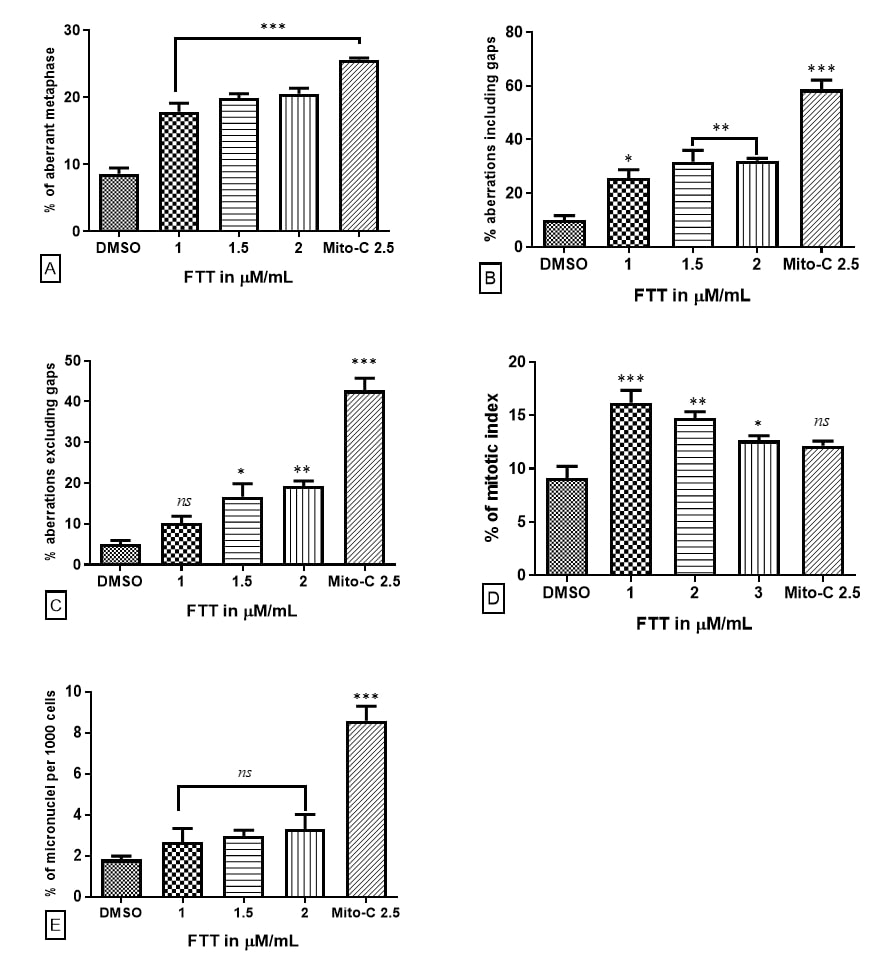
The CHO-K1 cells treated with three different concentrations of FTT induced higher percentage of chromosome aberrations compared to vehicle control. FTT at the lowest concentration (1 µM) was found to induce 56 aberrant metaphases (17.88%) out of 315 metaphases examined and 80 aberrations with 25.39 % and 10.15 % of aberrations including and excluding gaps respectively. The percentage of aberrant metaphases and aberrations including gaps were found statistically significant at (P<0.001) level and (P<0.05) respectively when compared with that of the vehicle control, DMSO. Whereas, percentage of aberrations excluding gaps was found statistically non-significant when compared with that of vehicle control. The intermediate concentration of FTT (1.5 µM) treated CHO-K1 cells exhibited 61 aberrant metaphases (19.89%) out of 307 metaphases and 97 aberrations with (31.59%) aberrations including gaps and (17.26%) aberrations excluding gaps. The percentage of aberrant metaphases was found statistically significant at (P<0.001) level whereas percentage of aberrations including and excluding gaps was found statistically significant at (P<0.01) and (P<0.05) levels respectively in comparison to that of DMSO. In the highest concentration, 2 µM concentration of FTT treated CHO-K1 cells induced 62 aberrant metaphases (20.52%) out of 302 metaphases examined and 97 aberrations with (32.11%) of aberrations including gaps and (19.20%) of aberrations excluding gaps. Percentage of aberrant metaphases was found statistically significant at (P<0.001) level when compared to that of vehicle control whereas both the percentage of aberrations including and excluding gaps were statistically significant at level (P<0.01) when compared to that of the vehicle control, DMSO (Table 1).
Mitotic index (MI)
Two thousand cells in triplicates were randomly screened for DMSO, three concentrations of FTT and Mitomycin-C after 24 hr post-treatment to CHO-K1 cells to calculate the rate in the changes in the dividing cells. The cell group treated with DMSO exhibited 277 dividing cells (9.12%) out of 6,039 total number of cells whereas, the positive control Mitomycin-C showed 371 (12.15%) dividing cells out of 3,051 cells. Although, the mitotic index induced by Mitomycin-C was found higher but statistically not significant when compared to DMSO (Table 2).
|
Test chemicals
|
Dose (µM) |
Total number of cells |
Total Number of dividing cells observed |
Percentage of Mitotic index |
|
DMSO (Vehicle control) |
1% |
3035 |
277 |
9.12 ± 1.12 |
|
FTT |
1 |
3448 |
558 |
16.18 ± 1.15*** |
|
1.5 |
3186 |
488 |
15.31 ± 0.58** |
|
|
2 |
3142 |
424 |
13.49 ± 0.45* |
|
|
Mitomycin-C (Positive control) |
2.5 |
3051 |
371 |
12.15 ± 0.42 (ns) |
|
±: Standard error of mean; *** P<0.001; **P< 0.01, * P< 0.05 (Significant level compared to vehicle control (1% DMSO) using Dunnett's Multiple Comparison Test |
||||
The cells treated with three test doses of FTT (1 µM, 1.5 µM and 2 µM) induced 558 dividing cells out of 3,448 (16.18 %), 488 dividing cells out of 3,186 (15.31%) and 424 dividing cells out of 3142 (13.49%) respectively. The resultant data on mitotic index revealed that FTT induced significant decrease in mitotic index with increase in the concentration exposed. The effects of lowest and intermediate concentrations i.e., 1 µM and 1.5 µM were found statistically significant at (P<0.001) and (P<0.01) respectively whereas, the highest concentration 2 µM was found statistically significant at (P<0.05) level when compared with that of Vehicle control, DMSO. However, the data suggests that FTT is non-mitotoxic towards cellular proliferation at any of the tested concentrations (Table 2).
Micronuclei (MN)
CHO-K1cells treated with DMSO exhibiting 11 micronuclei (1.82%) out of 6,039 binucleated cells which was found statistically not significant (Table 4). Mitomycin-C induced 52 micronuclei (8.60%) out of 6046 cells and was found statistically significant at (P<0.001) level when compared with that of vehicle control. Among the different concentrations of FTT, at 1 µM concentration induced 16 micronuclei (2.66 %) out of 6,006 cells, at concentration 1.5 µM it induced 18 micronuclei (2.96 %) out of 6,074 cells and at the highest concentration 2 µM, it exhibited 20 micronuclei (3.31%) out of 6,028 cells scored. On the whole, all the concentrations of FTT were found statistically non-significant at when compared with that of vehicle control group, DMSO. Based on the values obtained it was understood that FTT at any three tested concentration is not aneugenic in nature (Table 3).
|
Test chemicals |
Dose (µM) |
Total number of nucleated cells scored |
Number of micronucleus observed |
Percentage of micronuclei /1000cells |
|
DMSO (Vehicle control) |
1% |
6039 |
11 |
1.82 ± 0.16 |
|
FTT |
1 |
6006 |
16 |
2.66 ± 0.67(ns) |
|
1.5 |
6074 |
18 |
2.96 ± 0.29 (ns) |
|
|
2 |
6028 |
20 |
3.31 ± 0.71 (ns) |
|
|
Mitomycin-C (Positive control) |
2.5 |
6046 |
52 |
8.60 ± 0.70*** |
|
±: Standard error of mean; *** P<0.001 (Significant level compared to vehicle control (1% DMSO) using Dunnett's Multiple Comparison Test |
||||
Cell cycle studies using FACS in CHO-K1 cells
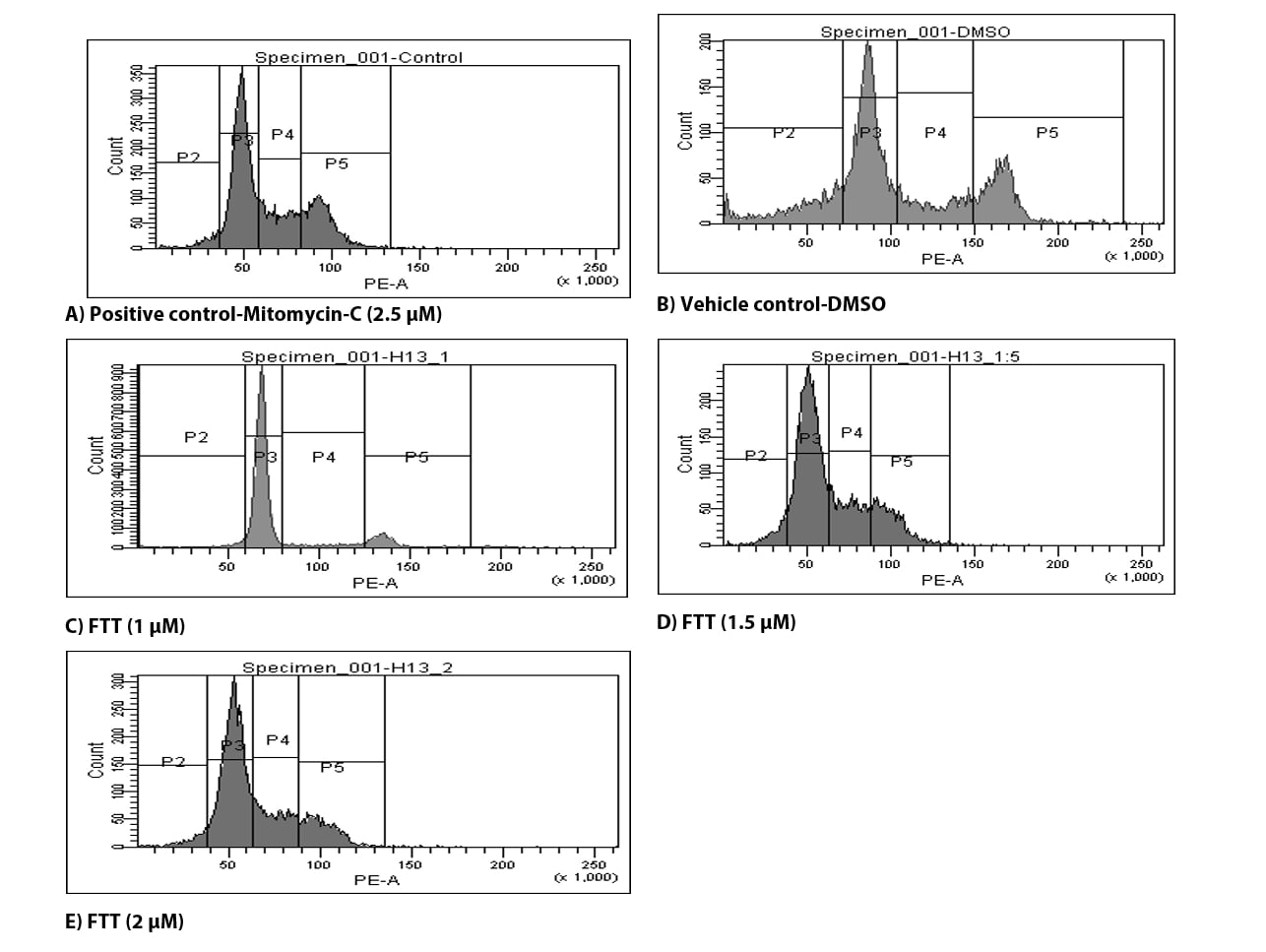
We investigated the effects of three selected sub-toxic concentrations of FTT on the regulation of cells at different phases of the cell cycle. Table 4 and Figure 4 show the typical cell cycle distribution of CHO-K1 cells with DMSO; representing4% of cells in Sub-G1 phase. Interestingly CHO-K1 cells with three test concentrations of FTT (1 µM, 1.5 µM and 2 µM) resulted in more or less equal percentage of cells in Sub-G1 with 4.3 %, 5.3 % and 4.4 % respectively. It was understood that the percentage of cells driven into apoptosis was negligible by FTT and the compound was minimally toxic. Whereas, high population of cells was found to block in G1/G0 phase, by all three tested concentrations of FTT when compared to that of vehicle control DMSO. This presence of high population of cells in G1/G0 phase and corresponding decrease of cells in following S and G2/M phases suggests that the compound caused G1 arrest and ceased the DNA synthesis of affected cells.
|
Test chemicals |
Concentration (µM) |
Cell cycle distribution (%) |
|||
|
Sub-G1 |
G1/G0 |
S |
G2/M |
||
|
DMSO (Vehicle control) |
1% |
4 |
49.1 |
21.7 |
24.9 |
|
FTT |
1 |
4.3 |
75.5 |
6.8 |
11.9 |
|
1.5 |
5.3 |
55.4 |
21.2 |
17.9 |
|
|
2 |
4.4 |
56.2 |
22.0 |
17.1 |
|
|
Mitomycin-C Positive control |
2.5 |
17.8 |
45.6 |
15.3 |
21.1 |
In addition, when the cell distribution was noticed in a dose-wise manner, accumulation of cells in S-phase and G2/M phases was increasing with increase in concentration. It was noticed following a G1 arrest, specifically with the higher concentrations of FTT (1.5 µM and 2 µM). In contrast, lower dose (1 µM) showed only 6.8 % of cells showed no such accumulation of cells in S-phase.
Discussion
Genotoxicity testing determines drug safety evaluation before their clinical use [10]. Hence, the main aim of this work was to establish an informative in vitro toxicological data of Fluorinated Trifluoromethyl 4-Thiazolidinone (FTT), which may retrieve useful information regarding the safety of this drug. The colorimetric tetrazolium salt (MTT) assay, which monitors metabolism inside cultured cells, analyzes the viability of cells exposed with the drugs [11]. In our previous studies, we observed that Cervical (SKOV3) and leukemia (HeLa) cancer cells showed potent anticancer effect with IC50 value with 4.62 and 2.52 µM respectively [5]. Then to analyze normal cell toxicity, CHO-K1 cells were treated with a series of increasing concentrations of FTT. It was found that 24 hr exposure to (1 to 5 µM) of FTT reduced the viability of CHO-K1 cells, with the most severe effect observed IC50 at 4.53 µM (Figure 2). The resultant data revealed that the compound possessed a potent cytotoxic nature upon both cancer and normal cells. In order to explore the DNA damaging effect of the FTT on normal cells, three sub-toxic concentrations (1 µM, 1.5 µM and 2 µM) were chosen and studied employing different cytogenetic toxicity methods.
Figure 2. Effect of FTT on the % of cell death in CHO-K1 cells.
Cytotoxicity of FTT in CHO-K1 cells
The purpose of the chromosomal aberration test is to identify agents that cause structural chromosome aberrations in cultured mammalian cells. All the three concentrations of FTT showed a significant increase in number of aberrant metaphases when compared to the vehicle control group (DMSO) with statistical significance of (P<0.001) level. The chromosome aberrations were mostly with chromatid types of gaps or breaks that are generally repaired in the subsequent cell division. In assessing the total number of aberrations induced by all the three sub-lethal concentrations of FTT, percentage of aberrations including gaps increased with increase in concentration and were shown significance at 1 µM (P<0.05), 1.5 µM (P<0.01) and 2 µM (P<0.01) respectively. Whereas, the percentage of aberrations excluding gaps too also increased with increase in concentration of FTT and were found statistically significant in comparison to DMSO. On the whole, all the three doses of FTT showed an increase in the number of chromatid and chromosome types of gaps and breaks. Hence the data reveals that the compound is clastogenic.
In the mitotic index analysis, FTT did not reduce the mitotic cell division after 24 hr post-treatment. A successful DNA repair always allows the cells to proceed with the normal cell cycle (not driving them into apoptosis but allowing to repair and mitotically divide). As the cells were found to undergo G1 arrest in the 24hr cell cycle analysis, it was understood that cells were able to repair the DNA damages rather than entering into apoptosis and fall into their normal cell cycle to divide mitotically in a healthier way. However, highly significant numbers of mitotic dividing cells were observed with 1 µM (P<0.001), at 1.5 µM (P<0.01) and 2 µM (P<0.05) of FTT comparing to the vehicle control group. Interestingly all these three concentrations also showed more number of dividing cells in comparison to the positive control group.
Aneuploidy is the presence of an abnormal number of chromosomes in a cell, due to an extra chromosome or a missing chromosome [12]. Aneuploidy originates during cell division whenever a whole chromosome or a fragment of a chromosome is not incorporated into one of the daughter nuclei. MN test is used as a tool to confirm the aneugenic (numerical anomaly) and clastogenic (structural anomaly) nature of a test compound [13]. In our study, numerical anomalies were noticed while counting the number of chromosomes in each metaphase plate. In addition, endoredupicated/polypoid cells were not noticed during the chromosome evaluation from all the tested concentrations of FTT. This shows that the micronuclei formed, may not be because of whole lagging chromosomes but due to fragments or broken isolated chromatid arms lagging behind the participation in anaphase of mitosis. In the chromosome aberration study, we also observed very lesser number of chromatid or chromosome types of breaks. Mostly, the chromatid breaks, fragments and minutes in presence of a toxicant generally induce the micronucleus formation in the subsequent cell division. However, the micronuclei induced by the structural damages were not found significant. The result displayed slight induction of MN by all the three tested concentrations of FTT, but the whole induction was found non-significant in comparison to DMSO and the FTT was determined as non-aneugenic.
Cell cycle checkpoints serve as surveillance systems to interrupt cell cycle progression when damage to the genome or spindle is detected or when cells have failed to complete a preceding event [14]. Propidium iodide (PI) flow cytometric assay has been widely used to evaluate cells at various phases of cell cycle [15]. The effect of three sub-lethal concentrations of FTT on cell cycle phases of CHO-K1 cells were determined according to the protocol [9] and analyzed using BD Canto fluorescence system. Figures 4A-4E shows the typical cell cycle distribution of CHO-K1 cells with DMSO; representing 4% of cells in Sub-G1 phase. Interestingly CHO-K1 cells incubated 24 hr with the three different test concentrations of FTT (1 µM, 1.5 µM and 2 µM) resulted in almost similar percentage of apoptotic cells in Sub-G1 phase (4.3 %, 5.3 % and 4.4 %) respectively. By this it was understood that the percentage of cells driven into apoptosis was negligible by FTT and the compound can be described as minimally toxic (in inducing apoptosis). Whereas, high populations of cells were seen blocked in G1/G0 phase, by all three test concentrations of FTT when compared to the vehicle control, DMSO. This presence of high population of cells (in G1/G0 phase) and corresponding decrease/ depletion of cells in following S and G2/M phases suggests that the compound caused G1 arrest and ceased the DNA synthesis of affected cells. In the normal cell cycle a primordial response to DNA damage initially triggers G1 arrest, blocking the progression of cells from G1 phase to S phase. This intermission time in cell cycling is naturally acceptable, as it gives the cells an enough time of relief, to repair DNA damages. These results are in accordance with the chromosome aberration tests, where higher number of chromatid types of breaks and gaps were noticed at all the FTT treated CHO-K1 cells (Table 1).
Figure 3. Occurrence of cytogenetic toxicity at different concentration of FTT after 24 hrs post-treatment with in CHO-K1 cells; (A) percentage of aberrant metaphases; (B) total number of aberrations including gaps; (C) total number of aberrations excluding gaps; (D) mitotic index (E) induction of micronucleus *** P<0.001; **P<0.01; *P<0.05 compared to vehicle control (DMSO).
Figures 4(A-E). Frequency of cells undergone different phases in cell cycle after 24 hrs post treatment with different concentrations concentration of FTT (µM) in CHO-K1 cell lines.
In addition, when the cell distribution was noticed in a dose-wise manner, accumulation of cells in S-phase and G2/M phase was increasing with increase in concentration. It was noticed following a G1 arrest, specifically with the higher concentrations of FTT (1.5 µM and 2 µM). In contrast, lower dose (1 µM showed only 6.8 % in S-phase and 11.9 % in G2/M phase showed very low accumulation of cells in both the phases. This data demonstrates that, with the increase in concentration, the percentage of cells undergoing G1 arrest was slightly decreasing following a subsequent accumulation in S-Phase and G2/M phase, where the structurally aberrant chromosomes might be undergoing a repair mechanism because of which their corresponding presence was not seen in apoptotic phase. If DNA damage is repaired successfully, the signals release the cells from checkpoint block and drive them into their normal cell cycle. Taken together, the results reveal that FTT is minimally toxic, showing negligible apoptosis, causing G1 arrest and subsequent accumulation of cells into S and G2/M-phases, providing a necessary time for the cells to repair DNA damages and reproduce through succeeding cell divisions.
Conclusion
Conclusively, FTT was understood to be potently clastogenic, inducing significant structural DNA damages in the normal cells. Results from other tests clearly elucidate important factors about FTT, such that it is cytotoxic but relatively non-aneugenic, non-mitotoxic and non-apoptotic, revealing its minimal toxic nature towards normal cells. These features suggest that the FTT deserves further detail investigations as a novel drug candidate for the treatment of cancer.
Acknowledgment
Authors thankful to Director, IICT for providing facilities. Thanks for ICMR, New Delhi, to KJ and UBK to CSIR, New Delhi, for the award of fellowship. Acknowledge to SERB, Science and Engineering Research Board, Government of India for financial assistance under EMEQ (EEQ/2019/000326) to RMK. We thank the KIM department CSIR-IICT, Hyderabad for providing IICT communication No. (IICT/Pubs./2022/393).
Declaration
Ethical approval and consent to participate
There were no animal experiments or human subjects used in the study.
Consent for publication
All authors declare no conflict of interest.
Availability of data and materials
Not applicable.
Authors’ contributions
KJ, JM and LK involved in the in vitro studies, KJ and SM prepared the manuscript UBK and RMK synthesis and characterization of the molecule.
References
2. Vigorita MG, Ottana R, Monforte F, Maccari R, Trovato A, Monforte MT, et al. Synthesis and antiinflammatory, analgesic activity of 3, 3′-(1, 2-Ethanediyl)-bis [2-aryl-4-thiazolidinone] chiral compounds. Part 10. Bioorganic & Medicinal Chemistry Letters. 2001 Nov 5;11(21):2791.
3. Szychowski KA, Leja ML, Kaminskyy DV, Binduga UE, Pinyazhko R, Lesyk RB, et al. Study of novel anticancer 4-thiazolidinone derivatives. Chemico-Biological Interactions. 2017 Jan 25; 262:46-56.
4. Eaton DL, Gilbert SG. Principles of toxicology. Casarett & Doull’s Toxicology. The Basic Science of Poisons. CD Klaassen (ed). 2008:11-34.
5. Umesh BK, Jhansi M, Tulshiram LD, Choudante PC, Suraj NM, Sunil M, et al. Synthesis of novel Thiazolidine-4-One derivatives, their cytotoxicity, antifungal properties, molecular docking and molecular dynamics. Russian Journal of Bioorganic Chemistry. 2023; (In press).
6. Javvaji K, Begum G, Deshpande SS, Rana RK, Misra S. Potential of the bioinspired CaCo3 microspheres loaded with tetracycline in inducing differential cytotoxic effects toward noncancerous and cancer cells: a cytogenetic toxicity assessment using CHO cells in vitro. Chemical Research in Toxicology. 2018 Jun 20; 31(7):629-36.
7. OECD. Guidance document on revisions to OECD genetic toxicology test guidelines. OECD Workgroup of National Coordinators for Test 42 Guidelines (WNT). 2015 Jan 9.
8. Deshpande SS, Veeragoni D, Rachamalla HK, Misra S. Anticancer properties of ZnO-Curcumin nanocomposite against melanoma cancer and its genotoxicity profiling. Journal of Drug Delivery Science and Technology. 2022 Sep 1; 75:103703.
9. Choudante PC, Nethi SK, Díaz-García D, Prashar S, Misra S, Gómez-Ruiz S, et al. Tin-loaded mesoporous silica nanoparticles: Antineoplastic properties and genotoxicity assessment. Biomaterials Advances. 2022 Jun 1; 137:212819.
10. Nath J, Krishna G. Safety screening of drugs in cancer therapy. Acta haematologica. 1998;99(3):138-47.
11. Berridge MV, Herst PM, Tan AS. Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnology Annual Review. 2005 Jan 1; 11:127-52.
12. Griffiths, AJF, Miller JH, Suzuki DT, Lewontin R, Gelbart WM. An introduction to genetic analysis. Seventh Edition.- WH Freeman and Company, New York. 2000; 867.
13. Luzhna L, Kathiria P, Kovalchuk O. Micronuclei in genotoxicity assessment: from genetics to epigenetics and beyond. Frontiers in Genetics. 2013 Jul 11; 4:131.
14. Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994 Dec 16; 266(5192):1821-8.
15. Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nature Protocols. 2006 Aug;1 (3):1458-61.