Abstract
Introduction: Histones play crucial roles in chromatin functioning and gene transcription, but they are incredibly harmful in intercellular space since they stimulate systemic inflammatory and toxic responses. Myelin basic protein (MBP) is the most meaningful protein of the axon myelin-proteolipid sheath. Antibodies with different enzymatic activities (abzymes) are substantial and specific features of some autoimmune diseases.
Methods: IgG preparations against individual histones (H1, H2A, H2B, H3, and H4) and MBP were obtained from the blood plasma of experimental autoimmune encephalomyelitis-prone C57BL/6 mice by several affinity chromatographies. These antibodies corresponded to different stages of experimental autoimmune encephalomyelitis (EAE) development: spontaneous, MOG (myelin oligodendrocyte glycoprotein), and DNA-histones complex accelerated onset, acute, and remission stages.
Results: IgG-abzymes against MBP and five individual histones demonstrated unusual complex formation polyreactivity and enzymatic cross-reactivity in the specific hydrolysis of histone H1. All IgGs of 3-month-old mice (zero time) against MBP and individual histones demonstrated from 4 to 23 different H1 hydrolysis sites. The spontaneous achievement of EAE during 60 days led to a powerful change in the type and number of H1 hydrolysis sites by IgGs against five histones and MBP. Treatment of mice with MOG and DNA-histones complex results in alteration in IgGs activities, a change in type, and an increase or decrease in the number of H1 hydrolysis sites compared to zero time. The minimum number (4) of different H1 hydrolysis sites was found for IgGs against ?2B (zero time) and ?2A (MOG treatment), while the maximum (26) – against H1 (MOG treatment).
Conclusion: Generally, it first demonstrated that at different stages of EAE evolution, IgG-abzymes against individual histones and MBP could significantly differ in specific sites and their number of H1 hydrolysis. Possible reasons for the catalytic cross-reactivity and strong differences in the number and type of cleavage sites are discussed.
Keywords
EAE model of human multiple sclerosis, C57BL/6 mice, Immunization mice with MOG and DNA-histones complex, Catalytic IgGs, Hydrolysis of histones and myelin basic protein, Cross-complexation and catalytic cross-reactivity
Abbreviations
Abs: Antibodies; AGDs: Antigenic Determinants; Abz: Abzyme; AA: amino acid; AIDs: Autoimmune Diseases; BFU-E: Erythroid Burst-Forming Unit (early erythroid colonies); CFU-GM: Granulocytic-Macrophagic Colony-Forming Unit; CFU-E: erythroid Colony-Forming Unit (late erythroid colonies); CFU-GEMM: Granulocytic-Erythroid-Megakaryocytic-Macrophagic Colony-Forming Unit; EAE: experimental autoimmune encephalomyelitis; ELISA: enzyme-linked immunosorbent assay; MBP: Myelin Basic Protein; MOG: Myelin Oligodendrocyte Glycoprotein; MS: Multiple Sclerosis; HIV-1: Human Immunodeficiency virus type 1; MALDI-TOF: Matrix-Assisted Laser Ionization/Desorption Times-of-Flight Mass Spectrometry; RA: Relative Activity; SLE: Systemic Lupus Erythematosus; SDS-PAGE: Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis
Introduction
Histones and their different modified forms are extremely important in chromatin functioning. Free extracellular histone molecules, however, usually act as damage factors [1]. Treatment of experimental mice with exogenous histones results in systemic toxic responses because of inflammatory reactions and activation of Toll-like receptors [1]. Treatment of mice with antibodies (Abs) neutralizing histones, activated protein C, heparin, and thrombomodulin leads to the mice protection against sepsis, trauma, ischemia-reperfusion injury, lethal endotoxemia, pancreatitis, peritonitis, stroke, coagulation, and thrombosis. Moreover, the increase in the free histones and fragments of nucleosomes in the blood results in several pathophysiological processes, including several autoimmune diseases (AIDs), progression in inflammatory processes, and cancer [1].
Tetramers in the core of nucleosome particles contain two molecules of H3 and H4 circled by two dimers of H2B and H2A histones bound with two supercoiled turns of double-stranded DNAs [2]. H1 histone is important for the packing chromatin substructures leading to a higher-order chromatin structure; H1 is first removed from chromatin during its treatment with acid or alkali.
In multiple sclerosis (MS) and systemic lupus erythematosus (SLE), as well as some other AIDs, antibodies to DNA are directed against histones-DNAs nucleosomal complexes appearing in the blood due to apoptosis of cells [3].
MS is an inflammatory-demyelinating autoimmune pathology of the central nervous system. It is characterized by large macrophages and T lymphocytes in perivascular infiltration [4]. The activated myelin-reactive CD4+ T cells might be the principal mediators of MS [4]. Some data is witnessed in MS pathogenesis about the vital role of B cells and auto-antibodies against autoantigens of myelin, including myelin basic protein (MBP) [4-6].
There are several mice models of experimental autoimmune encephalomyelitis (EAE) that well mimic a specific facet of human MS (for review, see [7,8]). Autoimmune diseases were first suspected may be originated from specific defects of bone marrow hematopoietic stem cells (HSCs) [9]. Later it was proved that the spontaneous and antigen-induced evolution of AIDs occurs due to specific autoimmune reorganization of bone marrow HSCs [10-16]. C57BL/6 mice prone to EAE were used for the study of possible mechanisms of spontaneous, accelerated with myelin oligodendrocyte glycoprotein (MOG) [12,13] and DNA-proteins complexes [10,11] development of EAE. It was shown that immunization of SLE-prone MRL-lpr/lpr mice with DNA-proteins complexes [14-16] and C57BL/6 mice with DNA-histones complexes or MOG [10-13] leads to a significant acceleration of SLE and EAE development. The acceleration is conditioned by specific changes in HSCs differentiation profiles, a significant increase in lymphocyte proliferation in parallel with repression of apoptosis in various organs of mice [10-16]. In addition, these changes are bound with the production of different auto-Abs-abzymes (Abzs) degrading polysaccharides, DNAs, RNAs, peptides, and proteins. The detection of various different auto-abzymes is the earliest and most statistically significant marker of the beginning of many AIDs [10-23]. Catalytic activities of abzymes are well detected even at the onset of several autoimmune pathologies (the pre-disease stage or the beginning of AIDs) before discovering typical markers of different AIDs [12-23]. Titers of auto-Abs to specific auto-antigens at the onset of many AIDs usually correspond to typical indices' ranges corresponding to those for healthy humans and mice. The appearance of plural Abzs clearly indicates the start of autoimmune reactions when an elevation in catalytic activities of Abs is associated with the development of profound pathologies. However, several parallel mechanisms can provide different AIDs development, eventually leading to self-tolerance breakdown [20-23].
Natural auto-abzyme splitting various oligosaccharides, peptides, proteins, nucleotides, DNAs, and RNAs were revealed in the blood sera of patients with several AIDs and viral diseases [18-23]. Auto-abzymes with feeble activities degrading polysaccharides [24], thyroglobulin [25,26], and vasoactive neuropeptide [27] were found in the blood of some healthy volunteers. However, conditionally healthy humans usually lack Abzs [18-23]. Notwithstanding, some germline auto-antibodies of conditionally healthy people could possess high levels of some amyloid-, superantigen-, and microbe-directed activities [28,29].
Similar to SLE patients [22], the blood of MS patients contains Abzs hydrolyzing DNAs and RNAs [30-32], MBP [33-36], oligosaccharides [20-23], and histones [37]. Relative activities (RAs) of IgG-abzymes from the cerebrospinal fluids of patients with MS hydrolyzing polysaccharides, MBP, and DNA are, on average, from 30 to 60 times higher than those isolated from the blood of the same patients [38-40]. In various AIDs, abzymes splitting MBP can attack this protein-substrate in the myelin-proteolipid sheath of axons. They could, therefore, have a very detrimental role in MS, SLE, and other AIDs pathogenesis [18-23].
Abzymes splitting five histones (H1, H2A, H2B, H3, and ?4) are revealed in sera of MS [37], HIV-infected patients [41-46], and EAE-prone mice [11,47]. As noted above, five free extracellular histones act as damage molecules [1]. DNA-histones complexes are known as the most important auto-antigens in producing antibodies against DNA and histones [4], which are very toxic for mammals. They could penetrate through membranes of cells and nuclei, hydrolyze chromatin DNA, and induce apoptosis of cells [48-50]. Therefore, abzymes splitting MBP, DNA, and histones may be important in the pathogenesis of MS and other AIDs.
It is believed that the evolution of different AIDs can be associated with the infection of humans by various viruses and/or bacteria, including human herpesvirus, human endogenous retroviruses, and Epstein-Barr virus (for review, see [51-56]). At first, there may be the production of Abs against bacterial or viral compounds, which have a high homology with human proteins [55,56]. Later, due to the mimicry of some bacterial or viral proteins with those of humans, immune system violations might lead to the generation of auto-antibodies to human proteins and the evolution of AIDs. Moreover, the treatment of different autoimmune-prone mice with different antigens leads to a significantly higher incidence of abzymes production with higher enzymatic activities than in normal conventionally used mouse strains [57,58].
The unspecific complex formation of different proteins and enzymes with foreign ligand molecules is a widespread phenomenon [59-61]. The level efficiency of the correct selection of specific substrates by enzymes in the first stage of complex formation is usually at most 1-2 orders of magnitude [59-61]. It is subsequent changes in enzymes and substrates structures that lead to the stage of catalysis, which provides an increase in the reaction rate by 5-8 orders of magnitude for specific in comparison with non-specific substrates [59-61]. Therefore, enzymatic cross-reactivity in the case of classical enzymes is a very sporadic case [59-61]. Typically, canonical enzymes usually catalyze only one chemical reaction.
Non-specific complex formation of some proteins and other ligands with Abs against other ones disclosed by the enzyme-linked immunosorbent assay (ELISA) or affinity chromatographies is a widely distributed phenomenon known as antibodies polyspecificity or polyreactivity complexation [62-65].
Similar to canonical enzymes, abzymes against many different proteins usually split specifically only one specific antigen-protein and could not split many other control unspecific globular proteins ([18-23] and refs therein). It was first shown that anti-MBP abzymes of patients with several AIDs hydrolyze only MBP [33-37], while Abs against histones - only histones [41-44]. However, studies of catalytic antibodies have shown that the immune response to auto-antigen in AIDs is more complex and multifaceted than could be imagined based on classical immunology.
The catalytic cross-activity of any Abs-abzymes against various proteins has not been described until recently [23]. But by now, it was demonstrated that auto-IgGs of HIV-infected patients against MBP hydrolyze, specifically MBP and five H1-H4 histones and vice versa – total preparations of abzymes against five histones effectively split MBP [45,46]. Recently Abs against five histones and MBP corresponding to different spontaneous, MOG, and DNA-histones accelerated onset, acute, and remission stages of C57BL/6 mice EAE development were analyzed [47]. Such IgG-abzymes against five histones and MBP possess unusual polyreactivity in complexation and enzymatic cross-reactivity in the hydrolysis of H4 histone. Such chimeric Abzs with cross-catalytic reactivity could be very hazardous for developing many AIDs since Abs against five histones can hydrolyze MBP of nerve tissue shells.
It was interesting to what extent the phenomenon of enzymatic cross-reactivity between abzymes against MBP and histones is common for humans and animals with various AIDs. It was important to understand whether there is an unusual catalytic cross-reactivity of antibodies-Abzs against MBP and histones only in the case of H4 or also for other histones. In addition, the analysis of abzymes at different stages of EAE development by C57BL/6 mice, in principle, makes it possible to understand how the relative activity of abzymes and their substrate specificity with respect to MBP and individual histones can be changed depending on the stage of pathology.
Therefore, here we, for the first time, purified not only total polyclonal IgGs against five histones but also isolated antibodies against five individual histones and MBP corresponding to various stages of EAE development. We then analyzed the antibodies' relative catalytic activity and enzymatic cross-reactivity against each of the five individual histones and MBP in the hydrolysis of H1 histone. It was shown that abzymes of mice against every of five histones and MBP possess unusual polyreactivity in complexation and demonstrate catalytic cross-reactivity in the splitting of H1 histone. Moreover, it has been demonstrated that Abzs against each of the five histones (H1-H4) and MBP corresponding to different stages of EAE development can hydrolyze H1 histone with different efficiency and in various specific sites.
Methods
Materials and chemicals
All compounds used, an equimolar mixture of five H1-H4 histones and individual homogeneous H1 histone, were from Sigma (St. Louis, MO, USA). Protein G-Sepharose and Superdex 200 HR 10/30 columns were from GE Healthcare (New York, USA). Myelin basic protein was obtained from the Center of Molecular Diagnostics and Therapy (DBRC; Moscow, Russia). Affinity sorbents containing immobilized MOG and histones (total or individual) were prepared according to the standard manufacturer's protocol using BrCN-activated Sepharose (Sigma), the mixture of five histones or 5 individual histones and MBP. Mouse MOG35-55 oligopeptide was from EZBiolab (Heidelberg, Germany). All preparations used by us were free from any possible contaminants.
Experimental animals
3-month-old inbred C57BL/6 mice (zero time) were used by us recently to analyze possible mechanisms of spontaneous and antigen-induced development of EAE [11,12,47]. They were obtained using standard conditions free of pathogens in a special mouse vivarium of the Institute of Cytology and Genetics (ICG). All experiments with C57BL/6 mice have been implemented under the Bioethical Committee of the ICG protocols (number of the document - 134A of 07 September 2010), according to the humane principles of European Communities Council Directive: 86/609/CEE concerning experiments with animals. This Committee of the Institute supported the study. The weights, titers of Abs against histones and MBP, the concentration of the urine proteins - proteinuria, mg/mL, and some other indexes characterizing progress in EAE development were analyzed and described in [11-14,47].
Antibody purification
Polyclonal IgGs from the blood plasma of C57BL/6 mice (electrophoretically homogeneous) were first isolated by plasma proteins affinity chromatography on Protein G-Sepharose. Preparations of IgGs were additionally purified by FPLC (fast protein liquid chromatography-gel filtration) on Superdex-200 HR 10/30 column [11-14,47]. For additional purification after gel filtration, central parts of IgGs peaks were filtrated through filters (pore size 0.1 µm) as in [11-14,47].
Removal of all antibodies against five histones (H1-H4) from total IgG preparations was fulfilled using histone5His-Sepharose (5 ml) containing immobilized 5 H1-H4 histones. The column was equilibrated with buffer A (20 mM Tris-HCl, pH 7.5). After antibodies loading, the column was washed with A buffer to zero optical density (A280). Adsorbed antibodies possessing a low affinity for five histones were eluted using A buffer supplemented with NaCl (0.2 M). Then, high affinity for the histones IgGs were desorbed specifically from column using an acidic buffer (0.1 M glycine-HCl, pH 2.6). The IgGs fractions eluted from histone5His-Sepharose at loading and the column washing with buffer A (5 mL) were unified and used to purify IgGs against MBP by their chromatography on the 5 ml MBP-Sepharose column equilibrated with buffer A. After the MBP-Sepharose washing with A buffer to zero optical density (A280), adsorbed low affinity for MBP IgGs were eluted first by buffer A and then with the same buffer supplemented with NaCl (0.2 M). Then, anti-MBP Abs were eluted from the affinity sorbent using acidic Tris-Gly buffer (pH = 2.6), similar to histone5His-Sepharose. Then, this fraction of IgGs was named and used as anti-MBP Abs. Such IgGs preparations were obtained in the case of EAE-prone mice corresponding to different stages of development of EAE before (Con-aMBP-0d, Spont-aMBP-60d), after immunization of mice with MOG (MOG20-aMBP) and DNA-histones complex (DNA20-aMBP and DNA60-aMBP) (Tab1e 1).
For additional purifications of IgGs against 5 histones from possible hypothetical impurities of antibodies against MBP, the fractions eluted from histone5His-Sepharose were subjected to re-chromatography on MBP-Sepharose. IgGs eluted at the loading on MBP-Sepharose were named anti-5-histones IgGs. IgGs against 5 histones were separated from the blood plasma of mice corresponding to different stages of development of EAE before (Con-aH1-H4-0d, Spont-aH1-H4-60d), after immunization of mice with MOG (MOG20-aH1-H4-20) and DNA-histones complex (DNA20- aH1-H4 and DNA60- aH1-H4) (Tab1e 1).
Antibody purification against individual five histones.
All preparations against five histones, corresponding to different stages of EAE development, were used further to get IgGs preparations against each of the 5 individual histones. All of the preparations were applied sequentially first on H1-Sepharose containing immobilized H1. The fractions eluted at loading on this sorbent were then applied sequentially to the following 4 sorbents: H2A-Sepharose, H2B-Sepharose, H3-Sepharose, and H4-Sepharose. All affinity chromatographies were performed as in the case of histone5His-Sepharose and MBP-Sepharose. IgGs against H1 and H2A-H4 histones were specifically eluted from each of five affinity sorbents with buffer, pH 2.6. These IgG fractions were designated respectively as anti-H1, anti-H2A, anti-H2B, anti-H3, and anti-H4 IgGs with the pointing to which stage and which antigen they correspond to: Con (beginning of the experiment); Spon (spontaneous of EAE development); MBP (mice treated with MOG); DNA (mice treated with DNA-histones complex) (Table 1).
|
Zero time (control), beginning of experiments (3 mice-old mice) |
Different total IgGs |
Designation |
IgGs against individual histones |
Designation |
|
Zero time (control) beginning of the experiment, IgGs against 5 histones and MBP |
Con-aH1-H4-0d |
anti-H1 histone |
Con-aH1-0d |
|
|
anti-H2A histone |
Con-aH2A-0d |
|||
|
anti-H2B histone |
Con-aH2B-0d |
|||
|
Con-aMBP-0d |
anti-H3 histone |
Con-aH3-0d |
||
|
anti-H4 histone |
Con-aH4-0d |
|||
|
Spontaneous development of EAE during 60 days (without mice immunization at 3 mice of age)
|
Spontaneous development of EAE during 60 days; IgGs against 5 histones and MBP |
Spont-aH1-H4-60d |
anti-H1 histone |
Spont-aH1-60d |
|
anti-H2A histone |
Spont-aH2A-60d |
|||
|
anti-H2B histone |
Spont-aH2B-60d |
|||
|
Spont-aMBP-60d |
anti-H3 histone |
Spont-aH3-60d |
||
|
anti-H4 histone |
Spont-aH4-60d |
|||
|
IgGs corresponding to 20 days after mice immunization with MOG |
IgGs against 5 histones and MBP corresponding to 20 days after mice immunization with MOG |
MOG20- aH1-H4-20 |
anti-H1 histone |
MOG20-aH1 |
|
anti-H2A histone |
MOG20-aH2A |
|||
|
anti-H2B histone |
MOG20-aH2B |
|||
|
MOG20- aMBP |
anti-H3 histone |
MOG20-aH3 |
||
|
anti-H4 histone |
MOG20-aH4 |
|||
|
IgGs corresponding to 20 days after mice immunization with complex DNA-histones |
IgGs against five histones and MBP; 20 days after mice immunization with complex DNA-histones |
DNA20- aH1-H4 |
anti-H1 histone |
DNA20-aH1 |
|
anti-H2A histone |
DNA20-aH2A |
|||
|
anti-H2B histone |
DNA20-aH2B |
|||
|
DNA20- aMBP |
anti-H3 histone |
DNA20-aH3 |
||
|
anti-H4 histone |
DNA20-aH4 |
|||
|
IgGs corresponding to 60 days after mice immunization with complex DNA-histones |
IgGs against fice histones and MBP; 60 days after mice immunization with complex DNA-histones |
DNA60- aH1-H4 |
anti-H1 histone |
DNA60-aH1 |
|
anti-H2A histone |
DNA60-aH2A |
|||
|
anti-H2B histone |
DNA60-aH2B |
|||
|
DNA60- aMBP |
anti-H3 histone |
DNA60-aH3 |
||
|
anti-H4 histone |
DNA60-aH4 |
|||
|
*All these IgG preparations were used for the analysis of H1 histone hydrolysis |
||||
Proteolytic activity assay
Protease activity of all IgGs-abzymes was analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) using the reaction mixtures (10–18 mL) containing 20 mM Tris-HCl buffer (pH 7.5), 0.9–1.0 mg/ml MBP, mixture of five histones, or H1 histone, and 0.012 mg/mL IgGs against histones or anti-MBP (0.07-0.1 mg/mL) as described in [47]. All reaction mixtures of histones and IgGs were incubated during 3–14 h at 37°C. All reactions were stopped by adding of SDS to the 0.1 % final concentration. The efficiency of ?1 and MBP hydrolysis was analyzed by SDS-PAGE in 20% gel. All gels were colored using silver or Coomassie Blue. The relative protease activities of IgGs were evaluated from the decrease in relative intensity of protein bands corresponding to initial non-hydrolyzed H1 or MBP corresponding to these proteins incubation without Abs. A more detailed analysis of the hydrolysis of H1 by all obtained Abs was carried out using the MALDI-TOF spectrometry method.
MALDI-TOF analysis of histone hydrolysis
H1 histone was hydrolyzed during 0-20 h using all IgG preparations against MBP or 5 individual histones using conditions described above. The ascertainment of the H1 histone hydrolysis products was performed by the 337-nm nitrogen laser VSL-337 ND, 3 ns pulse duration of Reflex III system (Bruker Frankfurt, Germany). Small aliquots of reaction mixtures (1–2 µL) were used after reaction mixtures incubation at different times by MALDI mass spectrometry. Sinapinic acid was used as the matrix. To 1.7 µl of the matrixes and 1.7 µl of 0.2% trifluoroacetic acid, 1.7 µL of the solutions containing histone H1 were added, and 1.0-1.7 µl of the obtained mixtures were applied on the iron MALDI plates. For the analysis, they were air-dried. All MALDI spectra were calibrated using standards II and I calibrant mixtures of special oligopeptides and proteins (Germany, Bruker Daltonic) in the external and/or internal calibration mode. The analysis of molecular weights and specific sites of H1 hydrolysis by various IgGs was done using Protein Calculator v3.3 (Scripps Research Institute).
Statistical analysis
The results correspond to the average values (mean ± standard deviation) from 8-10 independent spectra for each preparation of IgGs against five individual histones and MBP.
Results
Choosing a model for enzymatic cross-reactivity analysis
Theoretically, the human immune system can produce up to 106 variants of Abs against one antigen with different properties [66]. The possibilities of enzyme immunoassay (ELISA) and affinity chromatography in studying the possible diversity of antibodies in the sera blood of healthy donors and patients with AIDs against external and internal specific antigens are very limited.
As was shown in many publications (for review, see [18-23]), only the analysis of the catalytic activities of antibodies allows the revealing of an exceptionally expanded diversity of antibodies against different individual antigens.
An approximate evaluation of the possible number of abzymes for one antigen and their diversity in terms of enzymatic properties was carried out in several works [67-73]. A cDNA library of light chains (κ-type) of antibodies from SLE patients and the phage display method were used to obtain monoclonal abzymes. The pool of phage particles was divided into ten peaks eluted from MBP-Sepharose by different concentrations of NaCl. Phage particles of one peak (0.5 M NaCl) were used to obtain individual colonies and isolation corresponding to them monoclonal light chains of antibodies (MLChs). [67-73]. MLChs corresponding to 22 out of 72 (~30%) randomly selected of 440 individual colonies possess MBP-hydrolyzing activity. Of the 22 MLCh preparations with a comparable affinity for MBP, 12 had metalloprotease activity, four were serine-like, and three were thiol-like proteases. Two MLChs demonstrated serine and metalloprotease activities combined in one active site and one three activities - these two proteases and DNase activity [67-73].
It is important that, unlike other antibody analysis methods, determining the optimal conditions for the manifestation of catalytic activity makes it possible to distinguish between antibodies with comparable affinity for the same antigen. It showed that all preparations of MLCh-abzymes differed greatly in relative activity, optimal concentrations of ??different metal ions (K+, Na+, Mg2+, Zn2+, Mn2+, Co2+, Ni2+, etc.), as well as pH optima [67-73].
The DNA sequences of these MLChs were analyzed, and they were identical (89-100%) to the germ lines of IgLV8 light chain genes of several described Abs [71-73]. Homology analysis of the MLChs protein sequences with those of several classical Zn2+- and Ca2+-dependent and human serine and thiol proteases was carried out. The analysis revealed MLChs protein sequences responsible for MBP binding, metal ion chelation, and catalysis. All of them turned out to be very close to those for serine-like and metal-dependent classical proteases [71-73].
It should emphasize that IgGs of all peaks eluted from MBP-Sepharose had MBP-hydrolyzing activities. If we take into account the average percentage of active abzymes in one of 10 peaks, which is approximately 30%, and the number of analyzed individual colonies, then the possible number of abzymes of only κ–type of light chains with MBP-hydrolyzing activity in the blood of SLE patients can be ≥ 1000. However, the MBP-hydrolyzing activity was shown to possess antibodies with kappa and lambda chains [20-23].
The evolution of EAE in EAE-prone C57BL/6 mice arises spontaneously. Immunization of mice with DNA-histones complexes [11,12] or MOG significantly accelerates the development of EAE [13,14]. After mice immunization with MOG and DNA-histones complex there are several stages of the EAE progress: the onset at 7-8, the acute phase at 18-20, and the remission stage later 25-30 days. The acceleration of pathology achievement is associated with specific changes in profile of bone marrow HSCs differentiation and the increase in lymphocyte proliferation [11-14]. These processes, in parallel, are bound with the production of lymphocytes synthesizing Abzs degrading DNAs, RNAs, MBP, MOG, and histones. The parameters characterizing all these specific changes in mice were investigated earlier in [10-16]. To analyze the enzymatic cross-reactivity of IgGs, we have chosen two antigens described earlier; C57BL/6 mice immunized with MOG [13,14] and DNA-histones complex [10,11]. Data relating to changes in the differentiation profile of HSCs before and after mice treatment with MOG and DNA-histones complex are presented in Supplementary data (Supplementary Figure S1). Data on the changes in the titers of Abs against DNA, MOG, and histones during the evolution of EAE are given in Supplementary Figure S2. The changes in the relative activities of abzymes in the hydrolysis of MBP, MOG, DNA, and histones during the EAE development are demonstrated in Supplementary Figure S3. One can see that in time development of spontaneous EAE, the increment in the relative amounts of four precursors of hemopoietic cells [CFU-E (erythroid burst-forming unit - late erythroid colonies), CFU-GM (granulocytic-macrophagic colony-forming unit), CFU-GEMM (granulocytic-erythroid-megakaryocytic-macrophagic colony-forming unit), and BFU-E (erythroid burst-forming unit early erythroid colonies)] in the bone marrow of C57BL/6 mice are relatively slow and gradual. Mice immunization with MOG and DNA-histones complex results in various overtime changes in the profile of stem cells differentiation. On the whole, EAE development accelerates in all cases.
Recently, we studied a possible catalytic cross-reactivity of total polyreactive IgGs of C57BL/6 mice against five histones (H1-H4, isolated using Sepharose containing five immobilized histones) as well as MBP in the hydrolysis of H4 histone [47]. It was shown that the treatment of mice with MOG and complex DNA with histones results in the hydrolysis of H4 histones in different sites. Moreover, the sites of H4 histone hydrolysis by IgGs against five histones and MBP significantly change depending on the different stages of development of EAE. In this work, an even more detailed analysis of the enzymatic cross-activity of IgGs against five histones and MBP was carried out using the H1 histone hydrolysis analysis.
For this purpose, IgGs against each of the five individual histones were, for the first time, isolated from the fraction of antibodies against all five histones. It was shown that the treatment of mice with MOG leads to the onset of the pathology by 7–8 days (the appearance of abzymes) and a sharp exacerbation in the acute phase at 18–20 days (maximum activity of abzymes). After treatment of mice with a DNA–histones complex, the first peak of EAE development activation is observed in 7–20 days, but the activity of abzymes increases more strongly in the period of remission stage from 30 to 60 days. Thus, the following groups of mice were used to purify and analyze total IgGs against 5 histones, individual histones, and MBP corresponding to different stages of EAE (Table 1).
Purification of antibodies
Purification of electrophoretically homogeneous IgG preparations (IgGmix; the mixtures of 7 plasma blood samples corresponding to each of the mice groups) using Protein G-Sepharose and FPLC gel filtration in drastic conditions (pH 2.6) was described previously [47]. After SDS-PAGE of IgGmix samples, proteolytic activity in the histones and MBP was detected only in one IgG protein band (Supplementary Figure S4).
IgGmix against five histones corresponding to each of the mice groups was purified earlier by chromatography on histone5H-Sepharose containing five immobilized histones [47]. Non-specifically bound and having low affinity to five histones, IgG fractions were first eluted with 0.2 M NaCl. Anti-histones specific IgGs having a high affinity for five histones were eluted with Tris-Gly buffer, pH 2.6 [47]. For additional purification of IgGs against 5 histones from potential impurities of Abs against MBP, the fraction from histone5H-Sepharose was passed through MBP-Sepharose. The fraction obtained at loading onto MBP-Sepharose was further used as anti-5-histones IgGs in this article for purification of IgGs against each of the 5 individual histones (Table 1).
The IgGmix fraction eluted at loading from histone5H-Sepharose was used to isolate anti-MBP IgGs by their chromatography on MBP-Sepharose. IgGs with low affinity for MBP-Sepharose were eluted using NaCl (0.2 M). Anti-MBP Abs were eluted using an acidic buffer (pH 2.6) as in [47]. For additional removal of anti-MBP IgGs against potential impurities of anti-histones IgGs, the fraction eluted from MBP-Sepharose was subjected to re-chromatography on histone5H-Sepharose [47]. The fraction of IgGs eluted from the histone5H-sorbent at the loading was named anti-MBP IgGs (Table 1).
A possible enzymatic cross-reactivity of anti-5-histone IgGs (from histone5-Sepharose) and anti-MBP IgGs (from MBP-Sepharose) was first analyzed. Figures 1A and 1B demonstrate hydrolysis of H1 histone with anti-5-histone IgGs and anti-MBP IgGs, while Figures 1C and 1D show hydrolysis of MBP by these IgGs.
This study analyzed the possibility of hydrolysis of histone H1 with specific IgG-abzymes against five individual histones and MBP. The efficiency of H1 and MBP splitting with various IgGs was calculated from the decrease in these proteins in the initial bands (lanes C, Figure 1) after incubation with antibodies compared to their content in control-incubation H1 histone without antibodies. After 12 h of H1 incubation with IgGs against histones and MBP, the relative content of H1 (20.7 kDa) and MBP (18.5 kDa) form decreased remarkably or significantly compared to the control experiment (lanes C). These data suggest that anti-histones and anti-MBP IgGs of mice possess the known phenomenon of antibodies' unspecific complex formation polyreactivity [62-65] and enzymatic cross-reactivity in MBP and histones hydrolysis. These data, however, cannot provide absolute proof of enzymatic cross-reactivity between IgG-abzymes against MBP and five histones because it cannot be ruled out that after their isolations by several affinity chromatographies, the obtained antibodies nevertheless could contain very small admixtures of alternative IgGs. More powerful evidence of enzymatic cross-reactivity could be achieved from a significant difference in the specific sites of H1 histone hydrolysis by IgGs against MBP and five histones.
Figure 1. SDS-PAGE analysis of H1 histone hydrolysis by IgGs-abzymes against five histones (A) and this histone with IgGs against MBP (B) as well as splitting myelin basic protein by IgGs against five histones (C) and IgGs-abzymes against MBP (D). Lanes C correspond to the histones (A and B) and MBP (C and D) incubated without IgGs. The mixtures of five histones or MBP with and without IgGs (0.03-0.1 mg/ml) were incubated for 12 h.
MALDI specters of H1 histone hydrolysis
As shown by the example of IgG-abzymes of HIV-infected patients against five histones, they hydrolyze all histones and MBP and vice versa [45,46]. It was shown that total polyclonal IgGs of C57BL/6 mice also hydrolyze all five histones and MBP [10-13]. As mentioned above, IgGs against five histones and MBP from C57BL/6 mice demonstrate cross-enzymatic activity in the hydrolysis of H4 histone splitting [47]. In addition, the sites of hydrolysis of H4 by these Abs significantly depend on the stage of EAE development.
Here, IgGs from mice corresponding to different stages of EAE development were isolated for the first time against five individual histones and MBP, and an analysis of their enzymatic cross-reactivity in the hydrolysis of H1 histone was carried out.
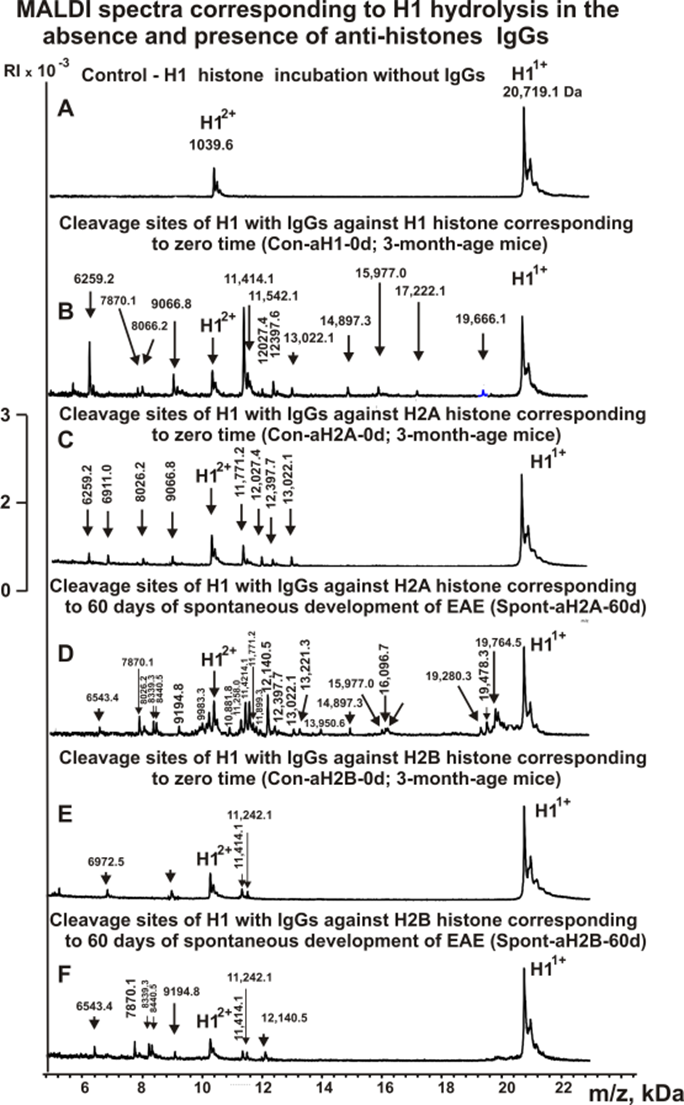
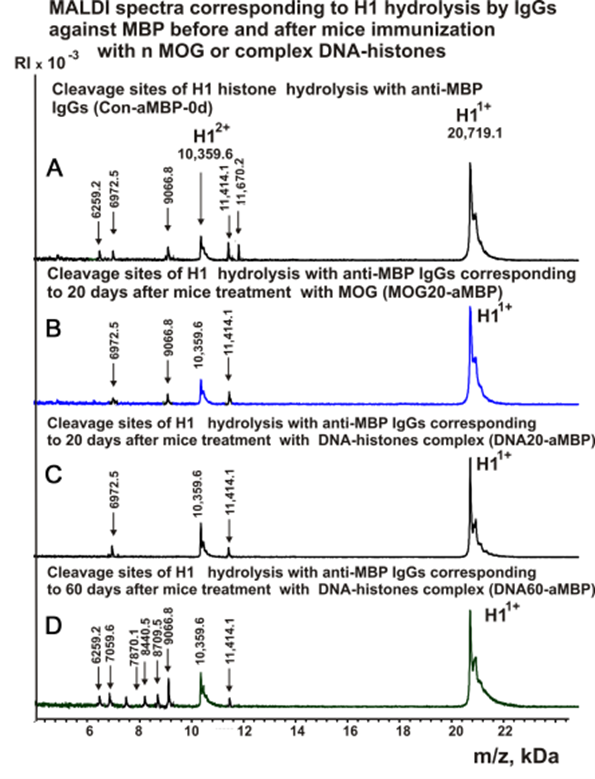
The IgG fractions having a high affinity to individual histones and MBP were used to reveal the cleavage sites of H1 by MALDI TOF mass spectrometry. First, an analysis of the hydrolysis sites of histone H1 was carried out using IgGs against five individual histones and MBP corresponding to the zero time of the experiment (3-months-old mice) and the spontaneous development of EAE (without immunization of mice) within 60 days (Figure 2). Right after the addition of the IgGs (Figure 2A), H1 histone was almost homogeneous, showing two signals of its one- (m/z = 20719.1 Da) and two-charged ions (m/z = 10359.6 Da). For all IgG preparations listed in Table 1, 8-10 spectra were obtained. Several typical specters are shown in Figure 2. Each preparation of IgGs demonstrated a specific set of peaks corresponding to the hydrolysis of H1 histone.
Figure 2. MALDI-TOF spectra corresponding to products of H1 (0.9 mg/ml) hydrolysis in the absence (A) and the presence of Abs (0.04 mg/ml) against five histones: Con-aH1-0d (B), Con-aH2A-0d (C), Spont-aH2A-60d (D), Con-aH2B-0d (E), and Spont-aH2B-60d (F). All designations of IgGs and the values of m/z are shown in the Figure.
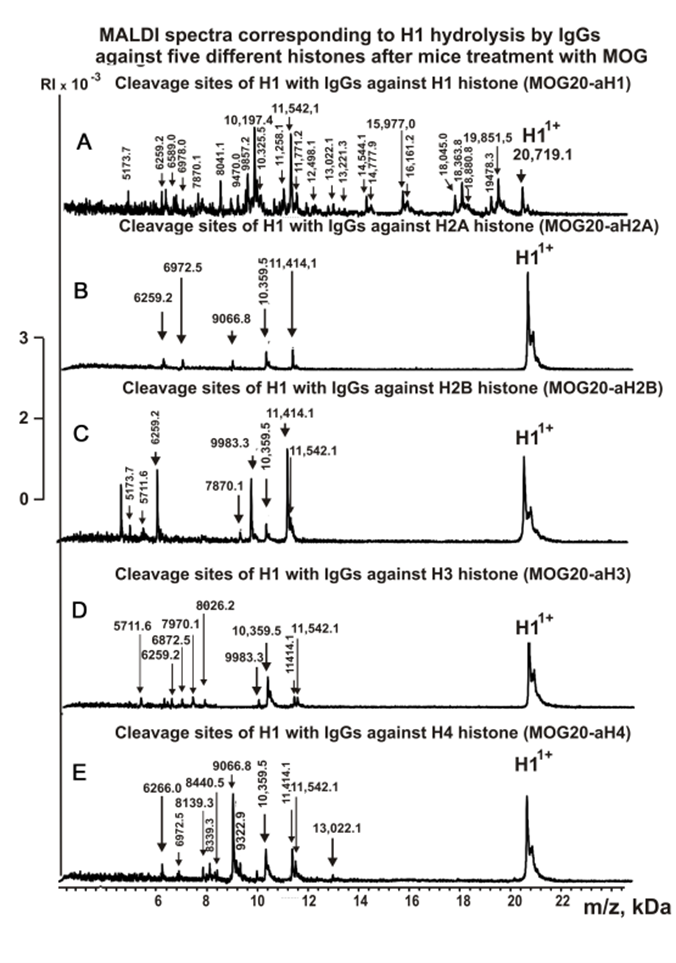
Figures 3-5 show spectra corresponding to hydrolysis of H1 by antibodies against various histones after immunization of mice with MOG and the DNA-histones complex.
Figure 3. MALDI spectra of H1 (0.9 mg/ml) hydrolysis products with IgGs against five histones corresponding to 20 days after mice immunization with MOG: MOG20-aH1 (A), MOG20-aH2A (B), MOG20-aH2B (C), MOG20-aH3 (D), MOG20-aH4 (E).
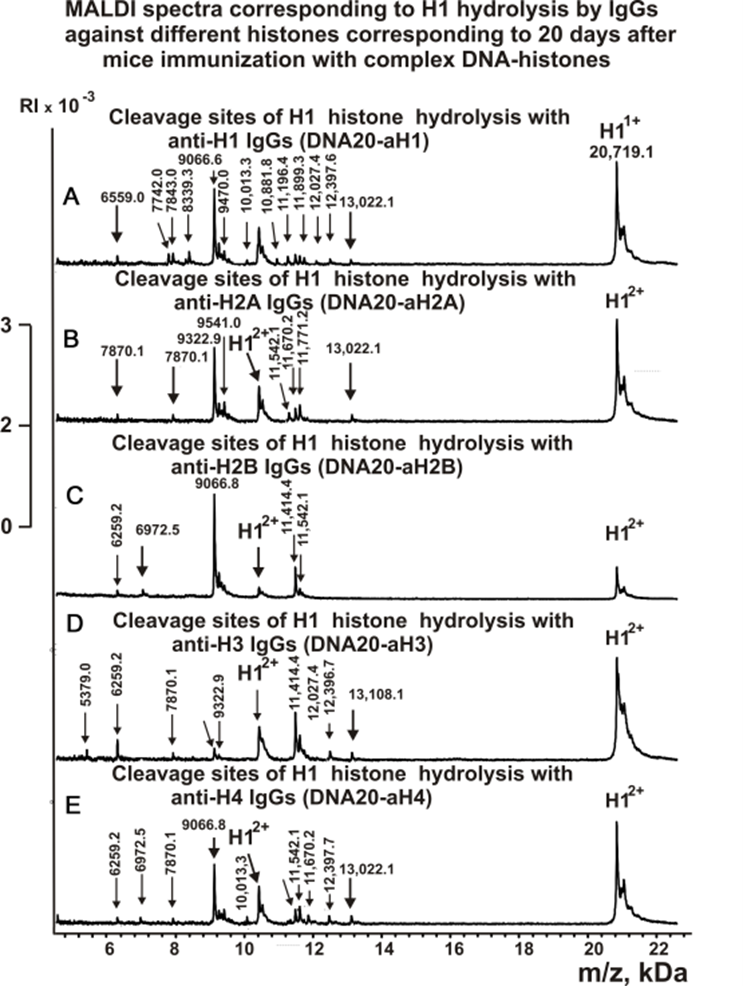
Figure 4. MALDI spectra of H1 (0.9 mg/ml) hydrolysis products with IgGs against five histones corresponding to 20 days after mice immunization with DNA-histones complex: DNA20-aH1 (A), DNA20-aH2A (B), DNA20-aH2B (C), DNA20-aH3 (D), DNA20-aH4 (E).
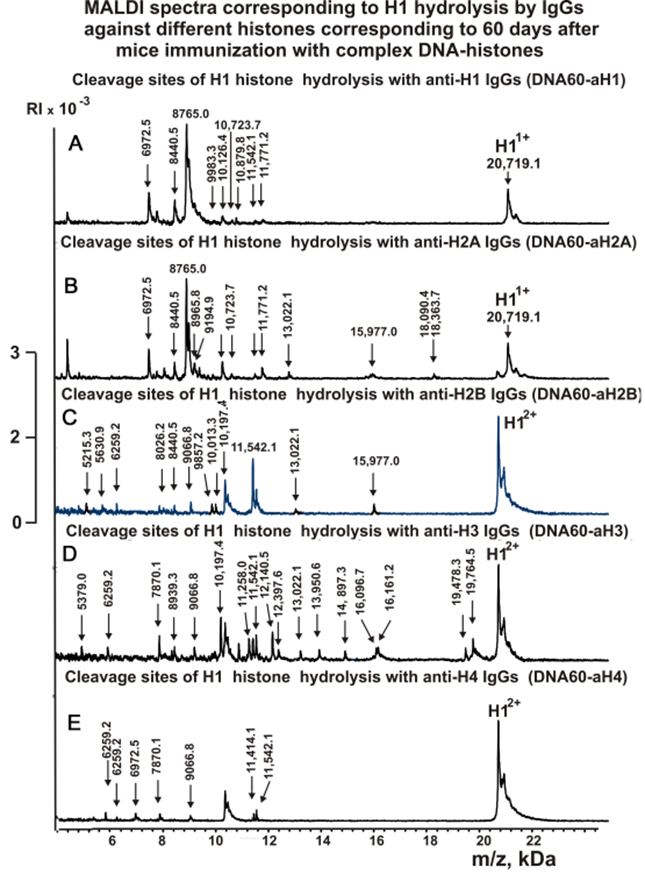
Figure 5. MALDI spectra of H1 (0.9 mg/ml) hydrolysis products with IgGs against five histones corresponding to 60 days after mice immunization with DNA-histones complex: DNA60-aH1 (A), DNA60-aH2A (B), DNA60-aH2B (C), DNA60-aH3 (D), DNA60-aH4 (E).
Figure 6 shows the spectra of H1 hydrolysis products with antibodies against MBP.
Figure 6. MALDI spectra of H1 (0.9 mg/ml) hydrolysis products with IgGs against MBP corresponding zero time and after mice immunization with MOG or DNA-histones complex: Con-a-MBP-0d (A), MOG20-aMBP (B), DNA20-aMBP (C), DNA60-aMBP (D).
As can be seen from Figures 2-6, each antibody preparation demonstrates its own specific set of peaks corresponding to hydrolysis products with different molecular weights.
Sites of H1 histone hydrolysis
The type of sites and relative efficiency of H1 hydrolysis by each of the IgG preparations were established based on an average data of 8-10 independent spectra. All sites of H1 histone hydrolysis by all IgG preparations against individual histones and MBP are summarized in Figures 7 -12.
Figure 7. Sites of H1 hydrolysis by IgG preparations against H1, H2A, and H2B three histones corresponding to 3-month-old mice (zero time) and after spontaneous development of EAE (before mice treatment) during 60 days: Con-aH1-0d (A); Spont-aH1-60d (B); Con-aH2A-0d (C); Spont-aH2A-60d (D); Con-aH2B-0d (E), Spont-aH2B-60d (F). Major sites of H1 histone cleavage are shown by stars (?), moderate ones by arrows (↓), and minor sites of the cleavages by small stars (*).
Figure 8. Sites of H1 hydrolysis by IgG preparations against H3 and H4 corresponding to 3-month-old mice (zero time) and after spontaneous development of EAE (before mice immunization) during 60 days: Con-aH3-0d (A), Spont-aH3-60d (B), Con-aH4-0d (C), Spont-aH4-60d (D). Major sites of H1 histone cleavage are shown by stars (?), moderate ones by arrows (↓), and minor sites of the cleavages by small stars (*).
Figure 9. Sites of H1 hydrolysis by IgGs against five H1 - H4 histones corresponding to 20 days after mice treatment with MOG: MOG20-aH1(A), MOG20-aH2A (B), MOG20-aH2B (C), MOG20-aH3 (D) and MOG20-aH4 (E). Major sites of H1 histone cleavage are shown by stars (?), moderate ones by arrows (↓), and minor sites of the cleavages by small stars (*).
Figure 10. Sites of H1 hydrolysis by IgGs against three H1, H2A, and H2B histones corresponding to 20 and 60 days after mice immunization with the complex of DNA and histones: DNA20-aH1(A), DNA60-aH1(B), DNA20-aH2A(C), DNA60-aH2A(D), DNA20-aH2B (E), DNA60-aH2B (F). Major sites of H1 histone cleavage are shown by stars (?), moderate ones by arrows (↓), and minor sites of the cleavages by small stars (*).
Figure 11. Sites of H1 hydrolysis by IgGs against H3 and H4 two histones corresponding to 20 and 60 days after mice immunization with DNA-histones complex: DNA20-aH3 (A), DNA60-aH3 (B), DNA20-aH4 (C), and DNA60-aH4 (D). Major sites of H1 histone cleavage are shown by stars (?), moderate ones by arrows (↓), and minor sites of the cleavages by small stars (*).
Figure 12. Sites of H1 hydrolysis by IgGs against MBP corresponding to zero time (3-month-old mice), 20 days after treatment with MOG as well as 20 and 60 days after mice immunization with DNA-histones complex: Con-aMBP-0d (A), MOG20-aMBP (B), DNA20-aMBP (C), and DNA60-aMBP (D). Major sites of H1 histone cleavage are shown by stars (?), moderate ones by arrows (↓), and minor sites of the cleavages by small stars (*).
Some data on the sites of H1 histone hydrolysis by IgG preparations against H3 and H4 histones are shown in Figure 8.
One can see that, overall, the sites of H1 histone cleavage by IgGs against H1-H4 five histones and MBP corresponding to 3-month-age mice (the beginning of the experiment), 60 days of spontaneous development of EAE, 20 days after mice immunization using with MOG, as well as 20 and 60 days after immunization with DNA-histones complex are substantially or very different and are predominantly located in specific amino acids (AAs) clusters of different lengths.
Tables with sites of H1 histone hydrolysis
All IgGs hydrolyze H1 histone in many various sites, the number of which also differs for different IgG preparations. For a more straightforward comparison of the different sites of hydrolysis of H1 with IgGs against three histones, they are given in Table 2.
|
Con-aH1-0d |
Spont-aH1-60d |
Con-aH2A-0d |
Spont-aH2A-60d |
Con-aH2B-0d |
Spont-aH2B-60d |
Con- aH3-0d |
Spont- aH3-60d |
Con- aH4-0d |
Spont- aH4-60d |
|
16 sites |
15 sites |
15 sites |
25 sites |
4 sites |
8 sites |
17 sites |
10 sites |
23 sites |
17 sites |
|
K11-P12** |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
R14-A15** |
- |
- |
- |
- |
- |
R14-A15 |
|
- |
- |
- |
- |
- |
- |
- |
- |
D23-H24 |
D23-H24** |
|
A33-A36 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
F33-A34 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
S44-S45 |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
- |
- |
- |
- |
Y52-I53 |
- |
- |
|
- |
- |
- |
- |
- |
- |
- |
- |
S55-H56 |
S55-H56 |
|
Y57-K58 |
Y57-K58 |
- |
- |
- |
- |
- |
Y57-K58 |
- |
- |
|
- |
- |
- |
- |
- |
- |
V59-G60 |
- |
V59-G60 |
V59-G60 |
|
- |
- |
- |
G60-E61 |
- |
G60-E61 |
G60-E61 |
- |
- |
- |
|
- |
- |
N62-A63 |
N62-A63 |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
- |
D64-S65 |
- |
- |
- |
- |
- |
|
- |
- |
- |
- |
- |
- |
I67-K68 |
- |
I67-K68 |
- |
|
- |
- |
- |
- |
- |
K68-L69 |
- |
- |
- |
- |
|
- |
- |
- |
- |
- |
- |
- |
- |
I71-K72 |
I71-K72 |
|
- |
- |
- |
K72-R73 |
- |
- |
- |
R73-L74 |
- |
- |
|
R73-L74 |
R73-L74 |
R73-L74 |
R73-L74 |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
- |
- |
- |
- |
- |
L74-V75 |
L74-V75 |
|
- |
- |
- |
- |
- |
- |
V75-T76 |
- |
V75-T76 |
V75-T76 |
|
T76-T77 |
T76-T77 |
- |
- |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
T77-G78 |
- |
T77-G78 |
- |
- |
- |
- |
|
- |
- |
- |
V79-L80 |
- |
V79-L80 |
- |
- |
- |
- |
|
L80-K81 |
L80-K81 |
L80-K81 |
- |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
K81-Q82 |
- |
- |
- |
- |
- |
- |
|
- |
Q82-T83 |
Q82-T83 |
Q82-T83 |
- |
- |
Q82-T83 |
- |
Q82-T83 |
Q82-T83 |
|
T83-K84 |
T83-K84 |
- |
- |
- |
- |
- |
- |
- |
- |
|
K84-G85 |
K84-G85 |
K84-G85 |
K84-G85 |
K84-G85 |
K84-G85 |
- |
- |
- |
- |
|
- |
- |
- |
- |
- |
- |
G85-V86 |
- |
- |
- |
|
V86-G87 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
A88-S89 |
- |
- |
- |
A88-S89 |
- |
- |
|
- |
F92-R93 |
- |
- |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
- |
- |
- |
R93-L94 |
- |
R93-L94 |
- |
|
- |
L94-A95 |
- |
- |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
D98-E99 |
- |
- |
D98-E99 |
- |
D98-E99 |
- |
|
- |
- |
- |
- |
- |
- |
- |
- |
E99-P100 |
- |
|
- |
- |
- |
K101-K102 |
- |
- |
K101-K102 |
- |
K101-K102 |
- |
|
- |
- |
- |
K102-S103 |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
- |
- |
- |
S103-V104 |
- |
S103-V104 |
S103-V104 |
|
- |
- |
- |
- |
- |
- |
V104-A105 |
- |
|
V104-A105 |
|
- |
A105-F106 |
- |
- |
- |
- |
A105-F106 |
- |
A105-F106 |
A105-F106 |
|
F106-K107 |
F106-K107 |
F106-K107 |
- |
F106-K107 |
F106-K107 |
- |
F106-K107 |
- |
- |
|
K107-K108 |
K107-K108 |
- |
K107-K108 |
- |
K107-K108 |
- |
K107-K108 |
- |
- |
|
K108-T109 |
K108-T109 |
K108-T109 |
- |
K108-T109 |
- |
- |
K108-T109 |
- |
- |
|
- |
- |
- |
- |
- |
- |
- |
- |
K110-K111 |
- |
|
- |
- |
- |
- |
- |
- |
E112-I113 |
E112-I113 |
E112-I113 |
|
|
- |
- |
- |
- |
- |
- |
- |
- |
- |
I113-K114 |
|
- |
- |
K114-K115 |
K114-K115 |
- |
K114-K115 |
- |
- |
- |
- |
|
- |
- |
- |
- |
- |
- |
K115-V116 |
- |
- |
- |
|
K120-K121 |
K120-K121 |
K120-K121 |
K120-K121 |
- |
- |
- |
- |
K120-K121 |
- |
|
- |
- |
- |
A122-S123 |
- |
- |
- |
S123-K124 |
- |
- |
|
- |
- |
- |
- |
- |
- |
- |
- |
K126-K127 |
K126-K127 |
|
- |
- |
- |
- |
- |
- |
A128-A129 |
- |
A128-A129 |
A128-A129 |
|
- |
- |
- |
K131-A132 |
- |
- |
- |
- |
- |
- |
|
K138-A139 |
K138-A139 |
- |
K138-A139 |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
- |
- |
- |
A139-T140 |
- |
A139-T140 |
A139-T140 |
|
- |
- |
- |
- |
- |
- |
K147-K148 |
- |
- |
- |
|
K148-L149 |
- |
- |
K148-L149 |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
A150-A151 |
- |
- |
- |
- |
A150-A151 |
A150-A151 |
|
- |
- |
- |
- |
- |
- |
- |
A151-T152 |
A151-T152 |
- |
|
- |
- |
- |
K170-A171 |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
- |
- |
- |
- |
- |
K181-A182 |
K181-A182 |
|
- |
- |
- |
S184-S185 |
- |
- |
- |
- |
- |
- |
|
*The molecular weights of H1 histone splitting products were used for estimation of the corresponding cleavage sites based on a set of data from 8-10 spectra.**Major sites of the hydrolysis are shown in red, moderate sites in black, and minor sites in green. Missing cleavage sites are marked with a dash (-). |
|||||||||
Antibodies against H1 histone of 3-months-old mice (zero time, Con-aH1-0d) hydrolyzed this histone at 16 sites, and after 60 days of spontaneous EAE development (Spont-aH1-60d) at only 15 sites; 9 out of 15-16 hydrolysis sites are different (Table 2).
During the spontaneous achievement of EAE, the number of histone H1 hydrolysis sites by Abs against H2A increases from 15 (Con-aH2A-0d) to 25 (Spont-aH2A-60d); 11 other major and moderate H1 histone hydrolysis sites appear (Table 2).
The number of sites of H1 hydrolysis by antibodies against H2B histone (Con-aH2B-0d) at zero time is minimal - only 4, but increases to 8 sites by 60 days (Spont-aH2B-60d); 4 new sites are major ones.
A somewhat different situation in H1 hydrolysis is observed for IgGs against H3 and H4 histones. The number of H1 hydrolysis sites for Spont-aH3-60d decreases in comparison with Con-aH3-0d from 17 to 10, and only one of these sites is the same for both IgGs (Table 2). The maximum number of H1 hydrolysis sites (23) at zero time was found for IgGs against H4 histone (Con-aH4-0d), which decreased to 17 by 60 days of spontaneous EAE (Table 2); five of a total of nine for two IgG preparations major sites of H1 hydrolysis are different (Table 2).
As shown by the example of the hydrolysis of five histones by IgGs against these histones from the blood of HIV-infected patients, the main reason for their cross-hydrolysis is the high level of homology of their protein sequences [45,46]. In addition, each histone has several antigenic determinants that can form complexes with antibodies against several homologous histones. The high level of homology of protein sequences of antigenic determinants of different histones can lead to the formation of specific complexes with cognate antibodies and less specific with other IgGs and, finally, to the hydrolysis of all histones not only by specific but also semi-specific abzymes.
When analyzing the obtained data, it should be taken into account that the development of AIDs occurs due to impaired differentiation of the stem cells of the bone marrow. Violation of the differentiation profile leads to the appearance in the cerebrospinal fluid and the blood of lymphocytes producing many different abzymes. In addition, there are at least two stages of HSCs changes; the first is related to the beginning and the acute phase of AIDs development, and the second to the remission stage [10-13]. It is important that every specific antigen stimulates not only the appearance of Abzs that hydrolyze only this antigen but also abzymes with other catalytic activities [18-23]. For example, immunization of C57BL/6 mice with MOG leads to the production of abzymes that hydrolyze MOG, MBP, DNA, and histones [18-23]. At the same time, treating mice using a DNA-histone complex also leads to the synthesis of abzymes hydrolyzing these four substrates [10-13]. In other words, each of these antigens can have an extended effect on the appearance of lymphocytes producing abzymes with different catalytic activities.
As shown in a number of publications [10-23], the appearance of abzymes in the blood is one of the earliest and most reliable indicators of the development of AIDs. The relatively high activity of abzymes in the blood of 3-month-old C57BL/6 mice indicates that autoimmune reactions can develop in EAE-prone mice at earlier stages, even before 3 months of life. At the same time, at different stages of the development of EAE, there may be developing lymphocytes that produce mainly IgGs to one or a limited number of all histones.
One of the important indicators of the specific characteristics of each abzyme is its major protein substrate hydrolysis sites. As can be seen from Table 2, IgGs-Abzs against five H1-H4 histones corresponding to different times of EAE development hydrolyze H1 at different sites. Remarkably, within 60 days of the spontaneous development of EAE, in the case of Abs against H2A and H2B, there is the increase, when for anti-H3 and anti-H4 IgGs, a decrease in the number of H1 splitting sites. Interestingly, for Abzs against all five histones corresponding to zero time and following 60 days of EAE development, there is no complete coincidence in terms of the number of splitting sites and also in terms of major sites of H1 hydrolysis. Antibodies against histone H3 are the brightest example of significant change in abzymes splitting sites during the spontaneous development of EAE. Interestingly, after 60 days, the number of sites of H1 hydrolysis by anti-H3 IgGs decreases from 17 to 10. At the same time, out of 10, only one coincides with the other 17 sites.
Most likely, within 60 days of the spontaneous EAE development, stimulation of the formation of various lymphocytes producing anti-H3 Abs-abzymes to different sequences of this histone can occur in comparison with zero time. Such specific abzymes against H3 of later periods of EAE development can hydrolyze H1 histone more efficiently and at other sites than IgGs of 3-month-old mice. In general, the data in Table 2 can speak in favor of very complex processes of regulation-switching of the differentiation profile of stem cells in the bone marrow of mice, leading to the development of lymphocytes synthesizing abzymes with very different properties.
As indicated above, immunization of C57BL/6 mice with MOG leads to a sharp acceleration of the EAE development up to 20 days (the acute phase with following remission of the pathology, > 25 days) [10-14]. It is during the acute phase was observed a very strong increase in the activity of abzymes hydrolyzing MOG, MBP, histones, and DNA. Therefore, we compared the hydrolysis of histone H1 by IgGs against five individual histones at zero time and 20 days after mice treatment with MOG (Table 3).
|
Con-aH1-0d |
MOG20-aH1 |
Con-aH2A-0d |
MOG20-aH2A |
Con-aH2B-0d |
MOG20-aH2B |
Con- aH3-0d |
MOG20-aH3 |
Con- aH4-0d |
MOG20-aH4 |
|
16 sites |
26 sites |
15 sites |
4 sites |
4 sites |
5 sites |
17 sites |
7 sites |
23 sites |
17 sites |
|
K11-P12** |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
- |
R14-A15 |
- |
- |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
A33-A36 |
D23-H24** |
- |
- |
- |
- |
- |
- |
- |
- |
|
F33-A34 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
|
S48-I49 |
- |
- |
- |
- |
- |
- |
- |
- |
|
- |
S55-H56 |
- |
- |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
- |
- |
H56-Y57 |
- |
- |
- |
- |
|
Y57-K58 |
- |
- |
- |
- |
- |
- |
- |
- |
Y57-K58** |
|
- |
- |
- |
- |
- |
- |
- |
- |
V59-G60 |
- |
|
- |
- |
- |
- |
- |
- |
G60-E61 |
- |
- |
- |
|
- |
- |
N62-A63 |
- |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
D64-S65 |
D64-S65 |
- |
- |
- |
- |
D64-S65 |
|
- |
- |
- |
- |
- |
- |
I67-K68 |
- |
I67-K68 |
- |
|
- |
- |
- |
- |
- |
K68-L69 |
- |
- |
- |
K68-L69 |
|
- |
- |
- |
- |
- |
- |
- |
- |
I71-K72 |
- |
|
- |
K72-R73 |
- |
- |
- |
- |
- |
K72-R73 |
- |
- |
|
R73-L74 |
- |
R73-L74 |
- |
- |
- |
- |
R73-L74 |
- |
- |
|
- |
- |
- |
- |
- |
- |
- |
- |
L74-V75 |
L74-V75 |
|
- |
V75-T76 |
- |
- |
- |
- |
V75-T76 |
- |
V75-T76 |
- |
|
T76-T77 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
- |
- |
- |
- |
- |
- |
T77-V78 |
|
L80-K81 |
- |
L80-K81 |
- |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
- |
Q82-T83 |
Q82-T83 |
- |
- |
- |
Q82-T83 |
- |
Q82-T83 |
Q82-T83 |
|
T83-K84 |
- |
- |
- |
- |
- |
- |
- |
- |
T83-K84 |
|
K84-G85 |
K84-G85 |
K84-G85 |
- |
K84-G85 |
K84-G85 |
- |
K84-G85 |
- |
K84-G85 |
|
- |
- |
- |
- |
- |
- |
G85-V86 |
- |
- |
- |
|
V86-G87 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
- |
A88-S89 |
- |
- |
- |
- |
- |
- |
- |
- |
|
- |
F92-R93 |
- |
- |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
- |
- |
- |
R93-L94 |
R93-L94 |
R93-L94 |
- |
|
- |
A95-K96 |
- |
- |
- |
- |
- |
- |
- |
- |
|
- |
K97-S98 |
- |
- |
- |
- |
- |
A95-K96 |
- |
- |
|
- |
- |
- |
- |
- |
- |
D98-E99 |
- |
D98-E99 |
- |
|
- |
- |
- |
- |
- |
- |
- |
- |
E99-P100 |
- |
|
- |
P100-K101 |
- |
- |
- |
P100-K101 |
- |
P100-K101 |
- |
- |
|
- |
K101-K102 |
- |
- |
- |
- |
K101-K102 |
- |
K101-K102 |
- |
|
- |
S103-V104 |
- |
- |
- |
- |
S103-V104 |
- |
S103-V104 |
S103-V104 |
|
- |
- |
- |
- |
- |
- |
V104-A105 |
- |
- |
- |
|
- |
A105-F106 |
- |
- |
- |
- |
A105-F106 |
- |
A105-F106 |
- |
|
F106-K107 |
- |
F106-K107 |
F106-K107 |
F106-K107 |
F106-K107 |
- |
F106-K107 |
- |
F106-K107 |
|
K107-K108 |
- |
- |
K107-K108 |
- |
- |
- |
- |
- |
K107-K108 |
|
K108-T109 |
|
K108-T109 |
|
K108-T109 |
|
- |
- |
- |
K108-T109 |
|
- |
- |
- |
-- |
- |
- |
- |
- |
K110-K111 |
- |
|
- |
- |
- |
- |
- |
- |
E112-I113 |
- |
E112-I113 |
- |
|
- |
|
K114-K115 |
|
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
- |
- |
- |
K115-V116 |
- |
- |
K115-V116 |
|
K120-K121 |
K120-K121 |
K120-K121 |
- |
- |
- |
- |
- |
K120-K121 |
K120-K121 |
|
- |
A122-S123 |
- |
- |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
- |
- |
- |
- |
- |
K126-K127 |
- |
|
- |
A128-A129 |
- |
- |
- |
- |
A128-A129 |
- |
A128-A129 |
- |
|
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
- |
K135-K136 |
|
- |
- |
- |
- |
- |
- |
- |
|
K138-A139 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
- |
A139-T140 |
- |
- |
- |
- |
A139-T140 |
- |
A139-T140 |
- |
|
- |
- |
- |
- |
- |
- |
K147-K148 |
- |
- |
- |
|
K148-L149 |
K148-L149 |
- |
- |
- |
- |
- |
- |
- |
- |
|
- |
A150-A151 |
- |
- |
- |
- |
- |
- |
A150-A151 |
- |
|
- |
A151-T152 |
- |
- |
- |
- |
- |
- |
A151-T152 |
- |
|
- |
K181-A182 |
- |
- |
- |
- |
- |
- |
K181-A182 |
- |
|
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
- |
S185-A186 |
- |
- |
- |
- |
- |
- |
- |
- |
|
*The molecular weights of H1 histone splitting products were used for estimation of the corresponding cleavage sites based on a set of data from 8-10 spectra.**Major sites of the hydrolysis are shown in red, moderate sites in black, and minor sites in green. Missing cleavage sites are marked with a dash (-). |
|||||||||
Immunization of mice with MOG led to an increase in H1 hydrolysis sites by antibodies against this histone from 16 (zero time) to 26 sites (Table 3). Remarkably, 4 new major sites were discovered, and some minor sites for Con-aH1-0d became moderate splitting sites in the case of MOG20-aH1 (Table 3). It is interesting that only five out of 26 sites of H1 hydrolysis by MOG20-aH1 antibodies (Table 3) coincided with five out of 15 sites of hydrolysis of this histone by Spont-aH1-60d antibodies (Table 2).
Treatment of mice with MOG led to a significant decrease in the sites of H1 hydrolysis by abzymes against histone H2A from 15 (Con-aH2A-0d, Table 2) to 4 (MOG20-aH2A, Table 3) sites. However, both Abs demonstrated the same major F106-K107 hydrolysis site. In the case of MOG20-aH2A (Table 3), one site of weak splitting coincided with one site of moderate H1 hydrolysis site for Spont-aH1-60d (Table 2), corresponding spontaneous development of EAE.
After immunization of mice with MOG, the number of H1 hydrolysis sites with IgGs against H2B increased compared to zero time from 4 to 5 sites (Table 3). All 4 sites of H1 hydrolysis by Con-aH2B-0d were minor, and 4 of the five splitting sites of this histone with MOG20-aH2B are major. One of the major sites in the case of Con-aH2B-0d was classified as minor for MOG20-aH2B, and vice versa, two minor sites for the first IgGs were identified as major sites for the second Abs (Tables 2 and 3).
Treatment of mice with MOG led to a decrease in the sites of H1 hydrolysis with IgGs against histone H3 from 17 (Con-aH2B-0d) to 7 (MOG20-aH3) sites (Table 3). At the same time, only one minor R93-L94 site was common in these two IgG preparations. In the case of MOG20-aH3 and Spont-aH3-60d, two of the same sites were revealed, and one of them (F106-K107) is major site for two preparations (Tables 2 and 3).
In the case of hydrolysis of H1 by IgGs against histone H4, the hydrolysis sites decreased from 24 (Con-aH4-0d) to 17 (MOG20-aH4) after mice immunization with MOG (Table 3). Only three sites of H1 hydrolysis by MOG20-aH4 and Spont-aH4-60d preparations are common but differ in the efficiency of H1 histone hydrolysis (Tables 2 and 3).
Overall, the immunization of mice with MOG leads to a strong increase in the number of H1 hydrolysis sites only in the case of IgGs against H1 histone. In other cases, except for IgGs against H2B, there was a tendency to decrease in the number of H1 hydrolysis by IgGs against the other 3 histones. But in all cases, sites of H1 hydrolysis by IgGs corresponding to zero time and spontaneous development of EAE within 60 days are significantly different from those after mice immunization with MOG (Tables 2 and 3). Moreover, it seems like immunization of mice with MOG resulting in a sharp increase in the activity of Abzs in the hydrolysis of MOG, MBP, and histones [10-13], leads to the different ways of changing the differentiation profile of bone marrow stem cells in comparison with that during the spontaneous development of this pathology.
Treatment of C57BL/6 mice with MOG and DNA-histone complex leads to acceleration of EAE development according to different changes in profiles of the differentiation of bone marrow stem cells. In addition, immunization of mice with MOG results in the production of antibodies with maximum DNase activity at 20 days, while with DNA-histone complex, the increase in this activity occurs in two stages - the first peak of activity is observed in 14-20 days, followed by a stronger increase in activity after 30 days [10,11]. Considering this, in order to compare the hydrolysis sites of H1 with IgGs against five histones at zero time and 20 days after treatment of mice with MOG, antibodies corresponding to 20 days after mice immunization with a DNA-histone complex were isolated and analyzed (Table 4).
|
Con-aH1-0d |
DNA20-aH1 |
Con-aH2A-0d |
DNA20-aH2A |
Con-aH2B-0d |
DNA20-aH2B |
Con- aH3-0d |
DNA20 aH3 |
Con- aH4-0d |
DNA20 aH4 |
|
16 sites |
16 sites |
15 sites |
13 sites |
4 sites |
5 sites |
17 sites |
9 sites |
23 sites |
10 sites |
|
K11-P12** |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
- |
- |
- |
- |
D23-H24** |
D23-H24** |
- |
|
A33-A36 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
F33-A34 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
- |
- |
- |
- |
- |
S55-H56 |
- |
|
Y57-K58** |
Y57-K58 |
- |
Y57-K58 |
- |
- |
- |
Y57-K58 |
- |
Y57-K58 |
|
- |
- |
- |
- |
- |
- |
V59-G60 |
- |
V59-G60 |
- |
|
- |
- |
- |
- |
- |
- |
G60-E61 |
- |
- |
- |
|
- |
- |
N62-A63 |
- |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
- |
D64-S65 |
D64-S65 |
- |
- |
- |
D64-S65 |
|
- |
- |
- |
- |
- |
- |
I67-K68 |
- |
I67-K68 |
- |
|
- |
I71-K72 |
- |
- |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
- |
- |
- |
- |
- |
I71-K72 |
- |
|
- |
- |
- |
K72-R73 |
- |
- |
- |
K72-R73 |
- |
K72-R73 |
|
R73-L74 |
- |
R73-L74 |
- |
- |
- |
- |
- |
- |
- |
|
- |
V75-T76 |
- |
- |
- |
- |
- |
- |
L74-V75 |
- |
|
- |
- |
- |
- |
- |
-- |
V75-T76 |
- |
V75-T76 |
- |
|
T76-T77 |
T76-T77 |
- |
- |
- |
|
- |
T76-T77 |
- |
- |
|
- |
- |
- |
- |
- |
V79-L80 |
- |
- |
- |
- |
|
L80-K81 |
L80-K81 |
L80-K81 |
- |
- |
- |
- |
- |
- |
- |
|
- |
K81-Q82 |
- |
- |
- |
- |
- |
L80-K81 |
- |
- |
|
- |
- |
Q82-T83 |
Q82-T83 |
- |
- |
Q82-T83 |
- |
Q82-T83 |
- |
|
T83-K84 |
T83-K84 |
- |
T83-K84 |
- |
- |
- |
T83-K84 |
- |
T83-K84 |
|
K84-G85 |
- |
K84-G85 |
K84-G85 |
K84-G85 |
K84-G85 |
- |
K84-G85 |
- |
K84-G85 |
|
- |
- |
- |
G85-V86 |
- |
- |
G85-V86 |
- |
- |
- |
|
V86-G87 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
A88 –S89 |
- |
- |
- |
- |
- |
- |
|
- |
R93-L94 |
- |
R93-L94 |
- |
- |
R93-L94 |
- |
R93-L94 |
R93-L94 |
|
- |
- |
- |
- |
- |
- |
D98-E99 |
- |
D98-E99 |
- |
|
- |
- |
- |
- |
- |
- |
- |
- |
E99-P100 |
- |
|
- |
K101-K102 |
- |
- |
- |
- |
K101-K102 |
- |
K101-K102 |
- |
|
- |
- |
- |
- |
- |
- |
S103-V104 |
- |
S103-V104 |
- |
|
- |
V104-A105 |
- |
V104-A105 |
- |
- |
V104-A105 |
- |
- |
- |
|
- |
A105-F106 |
- |
A105-F106 |
- |
- |
A105-F106 |
- |
A105-F106 |
- |
|
F106-K107 |
F106-K107 |
F106-K107 |
F106-K107 |
F106-K107 |
F106-K107 |
- |
F106-K107 |
- |
F106-K107 |
|
K107-K108 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
K108-T109 |
K108-T109 |
K108-T109 |
K108-T109 |
K108-T109 |
K108-T109 |
- |
K108-T109 |
- |
K108-T109 |
|
- |
- |
- |
- |
- |
- |
- |
- |
K110-K111 |
- |
|
- |
- |
- |
- |
- |
- |
E112-I113 |
- |
E112-I113 |
- |
|
- |
K114-K115 |
- |
- |
- |
- |
- |
- |
- |
K114-K115 |
|
- |
P119-K120 |
- |
- |
- |
- |
K115-V116 |
- |
- |
- |
|
K120-K121 |
K120-K121 |
K120-K121 |
K120-K121 |
- |
- |
- |
- |
K120-K121 |
K120-K121 |
|
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
- |
- |
- |
- |
- |
K126-K127 |
- |
|
- |
- |
- |
- |
- |
- |
A128-A129 |
- |
A128-A129 |
- |
|
K138-A139 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
- |
- |
- |
A139-T140 |
- |
A139-T140 |
- |
|
- |
- |
- |
- |
- |
- |
K147-K148 |
- |
- |
- |
|
K148-L149 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
- |
- |
- |
- |
- |
A150-A151 |
- |
|
- |
- |
- |
- |
- |
- |
- |
- |
A151-T152 |
- |
|
- |
- |
- |
- |
- |
- |
- |
- |
K181-A182 |
- |
|
*The molecular weights of H1 histone splitting products were used for estimation of the corresponding cleavage sites based on a set of data from 8-10 spectra.**Major sites of the hydrolysis are shown in red, moderate sites in black, and minor sites in green. Missing cleavage sites are marked with a dash (-). |
|||||||||
The total number of H1 hydrolysis sites by IgGs against H1 histone at time zero (16; Con-aH1-0d) and 20 days after immunization of mice with the DNA-histones complex (DNA20-aH1) turned out to be the same. At the same time, only 7 of 16 hydrolysis sites were the same for the two antibody preparations. The number of H1 hydrolysis sites after 20 days of the mice treatment of MOG (MOG20-aH1) was 26 and not 16 (Tables 3 and 4). It is interesting that only 4 common sites of H1 hydrolysis for these two antibodies (MOG20-aH1 and DNA20-aH1) are the same, and the first IgGs effectively hydrolyze H1 histone in its C-terminal zone (K120-A186), where there are no zone hydrolysis sites in the case of second antibodies.
The number of reliably registered H1 splitting sites by anti-H2A IgGs after mice immunization with DNA-histones complex decreased from 15 to 13 (Table 4), and only one major F106-K107 site is common for Con-aH2A-0d and DNA20-aH2A antibodies. At the same time, treatment of mice with MOG led to a decrease in the sites of hydrolysis of H1 by antibodies against H2A histone from 15 to 4 (Table 3), and only one (Y57-K58) of these four sites coincides with that for DNA20-aH2A antibodies (Table 4).
The number of H1 hydrolysis sites with IgGs against H2B (DNA20-aH2B) after immunization of mice with DNA-histone complex compared to zero time (Con-aH2B-0d) increased from 4 to 5, and four of them coincide (Table 4). But of the five H1 hydrolysis sites, only 2 are common for DNA20-aH2B and MOG20-aH2B antibodies (Tables 3 and 4). In other words, immunization of mice with DNA-histones complex weakens the development of antibodies against H2B capable of hydrolyzing H1 histone. Interestingly, the treatment of mice with MOG also had a weak effect on producing such antibodies (MOG20-aH2B; 5 sites).
By 20 days after the treatment of mice with the DNA-histone complex, the number of H1 hydrolysis sites by IgGs against histone H3 decreased from 17 to 9 (Table 4). A feature of IgGs against histone H3 is that all sites of the hydrolysis corresponding to zero time (Con-aH3-0d) and after immunization of mice with the DNA-histones complex (DNA20-aH3) are different. Also, a decrease in the number of H1 hydrolysis sites from 17 to 7 was observed after immunization of mice with MOG (MOG20-aH3; Table 3), and three of these seven sites coincided with three sites after mice immunization with DNA-histone complex (DNA20-aH3; Table 4).
The maximum number of H1 hydrolysis of sites (23) at zero time was observed for antibodies against H4 histones (Con-aH4-0d), which decreased after immunization of mice with a DNA-histone complex to 10 (DNA20-aH4; Table 4), and with MOG to 17 (MOG20-aH4; Table 3). Of the 10 sites for DNA20-aH4, seven were identical to those for MOG20-aH4 (Tables 3 and 4). Only 2 out of 10 sites of H1 degradation by DNA20-aH4 antibodies were the same as those for Con-aH4-0d (Tables 3 and 4).
The main immunogen for the development of Abs against DNA and histones in the development of AIDs are complexes of DNA with histones that appear in the blood as a result of cell apoptosis [3]. Interestingly, the activity of abzymes in the hydrolysis of histones after immunization of mice with a DNA-histones complex reaches its maximum at 20 days after immunization [10,11]. At the same time, the increase in DNase activity occurs mainly after 30 days after mice immunization with the DNA-histones complex. Considering this, it was interesting to compare how sites of H1 hydrolysis change during spontaneous development during 60 days and after immunization of mice with DNA-histones complex (Table 5).
|
Spont-aH1-60d |
DNA60-aH1 |
Spont-aH2A-60d |
DNA60-aH2A |
Spont-aH2B-60d |
DNA60-aH2B |
Spont- aH3-60d |
DNA60-aH3 |
Spont- aH4-60d |
DNA60-aH4 |
|
15 sites |
8 sites |
25 sites |
15 sites |
8 sites |
12 sites |
10 sites |
16 sites |
17 sites |
6 sites |
|
- |
- |
R14-A15** |
- |
- |
- |
- |
- |
R14-A15** |
- |
|
- |
- |
- |
D23-H24** |
- |
- |
- |
- |
D23-H24* |
- |
|
- |
- |
S44-S45 |
- |
- |
- |
- |
S44-S45 |
- |
- |
|
- |
- |
- |
- |
- |
Y52-I53 |
Y52-I53 |
- |
- |
- |
|
- |
- |
- |
S55-H56 |
- |
- |
- |
- |
S55-H56 |
- |
|
Y57-K58 |
- |
- |
- |
- |
Y57-K58 |
Y57-K58 |
Y57-K58 |
- |
Y57-K58 |
|
- |
- |
- |
- |
- |
- |
- |
- |
V59-G60 |
- |
|
- |
- |
G60-E61 |
- |
G60-E61 |
- |
- |
- |
- |
- |
|
- |
- |
N62-A63 |
- |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
D64-S65 |
- |
- |
- |
- |
- |
D64-S65 |
|
- |
|
- |
- |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
- |
K68-L69 |
- |
- |
- |
- |
- |
|
- |
- |
- |
- |
- |
- |
- |
- |
I71-K72 |
- |
|
- |
- |
K72-R73 |
|
- |
- |
- |
K72-R73 |
- |
K72-R73 |
|
R73-L74 |
- |
R73-L74 |
- |
- |
R73-L74 |
R73-L74 |
- |
- |
- |
|
- |
- |
- |
- |
- |
-- |
- |
- |
L74-V75 |
- |
|
- |
- |
- |
- |
- |
- |
- |
- |
V75-T76 |
- |
|
T76-T77 |
- |
- |
- |
- |
- |
- |
T76-T77 |
- |
- |
|
- |
- |
T77-G78 |
- |
T77-G78 |
T77-G78 |
- |
- |
- |
- |
|
- |
- |
V79-L80 |
- |
V79-L80 |
- |
- |
V79-L80 |
- |
- |
|
L80-K81 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
- |
- |
K81-Q82 |
- |
- |
- |
- |
- |
- |
- |
|
Q82-T83 |
Q82-T83 |
Q82-T83 |
Q82-T83 |
- |
- |
- |
- |
Q82-T83 |
- |
|
T83-K84 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
K84-G85 |
- |
K84-G85 |
- |
K84-G85 |
K84-G85 |
- |
K84-G85 |
- |
K84-G85 |
|
- |
- |
A88-S89 |
- |
- |
- |
A88-S89 |
A88-S89 |
- |
- |
|
- |
- |
- |
S91-F92 |
- |
- |
- |
- |
- |
- |
|
F92-R93 |
F92-R93 |
- |
F92-R93 |
- |
F92-R93 |
- |
- |
- |
- |
|
- |
R93-L94 |
- |
R93-L94 |
- |
R93-L94 |
- |
- |
- |
- |
|
L94-A95 |
L94-A95 |
- |
L94-A95 |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
- |
- |
A95-K96 |
- |
A95-K96 |
- |
- |
|
- |
- |
- |
K96-S97 |
- |
- |
- |
- |
- |
- |
|
- |
- |
D98-E99 |
- |
- |
- |
- |
- |
- |
- |
|
- |
P100-K101 |
- |
- |
- |
- |
- |
- |
- |
- |
|
- |
- |
K101-K102 |
- |
- |
- |
- |
K101-K102 |
- |
- |
|
- |
K102-S103 |
K102-S103 |
- |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
- |
- |
- |
- |
- |
S103-V104 |
- |
|
- |
- |
- |
- |
- |
- |
- |
- |
V104-A105 |
- |
|
A105-F106 |
A105-F106 |
- |
A105-F106 |
- |
- |
- |
- |
A105-F106 |
- |
|
F106-K107 |
- |
- |
- |
F106-K107 |
- |
F106-K107 |
F106-K107 |
- |
F106-K107 |
|
K107-K108 |
- |
K107-K108 |
K107-K108 |
K107-K108 |
- |
K107-K108 |
- |
- |
- |
|
K108-T109 |
- |
- |
- |
- |
K108-T109 |
K108-T109 |
- |
- |
K108-T109 |
|
- |
- |
- |
- |
- |
- |
- |
T109-K110 |
- |
- |
|
- |
- |
- |
- |
- |
- |
E112-I113 |
- |
|
- |
|
- |
- |
- |
- |
- |
- |
- |
- |
I113-K114 |
- |
|
- |
- |
K114-K115 |
- |
K114-K115 |
- |
- |
K114-K115 |
- |
- |
|
K120-K121 |
- |
K120-K121 |
K120-K121 |
- |
K120-K121 |
- |
- |
- |
- |
|
- |
- |
- |
K121-A122 |
- |
- |
- |
- |
- |
- |
|
- |
- |
A122-S123 |
- |
- |
- |
S123-K124 |
- |
- |
- |
|
- |
- |
- |
- |
- |
- |
- |
- |
K126-K127 |
- |
|
- |
- |
- |
- |
- |
- |
- |
- |
A128-A129 |
- |
|
- |
K131-A132 |
K131-A132 |
- |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
P133-T134 |
- |
- |
- |
- |
- |
- |
|
K138-A139 |
- |
K138-A139 |
- |
- |
- |
- |
K138-A139 |
- |
- |
|
- |
- |
- |
- |
- |
- |
|
|
A139-T140 |
- |
|
- |
- |
- |
T140-P141 |
- |
A145-K146 |
- |
- |
- |
- |
|
- |
- |
K148-L149 |
- |
- |
K148-L149 |
- |
- |
- |
- |
|
- |
- |
A150-A151 |
- |
- |
- |
- |
A150-A151 |
A150-A151 |
- |
|
- |
- |
- |
- |
- |
- |
A151-T152 |
- |
- |
- |
|
- |
- |
K170-A171 |
- |
- |
- |
- |
- |
- |
- |
|
- |
- |
- |
- |
- |
- |
- |
K181-A182 |
K181-A182 |
- |
|
- |
- |
S184-S185 |
- |
- |
- |
- |
S184-S185 |
- |
- |
|
*The molecular weights of H1 histone splitting products were used for estimation of the corresponding cleavage sites based on a set of data from 8-10 spectra.**Major sites of the hydrolysis are shown in red, moderate sites in black, and minor sites in green. Missing cleavage sites are marked with a dash (-). |
|||||||||
It is interesting that compared to the spontaneous development of EAE within 60 days, immunization of mice with a DNA-histones complex after 60 days leads to a rise in the number of H1 hydrolysis sites by antibodies against H2B (DNA60-aH2B) and H3 (DNA60-aH3) histones (Table 5). However, in the case of antibodies against three other histones, there was a decrease in sites of H1 hydrolysis.
Out of eight sites of H1 hydrolysis by anti-H1 IgGs (DNA60-aH1, only three coincide with three sites corresponding to spontaneous development of EAE - Spont-aH1-60d (Table 5). At zero time, 16 sites of hydrolysis of H1 by IgGs against histone H1 (Con-aH1-0d) were detected (Table 2). None of the 8 sites of H1 hydrolysis by IgGs against H1 after immunization of mice with the DNA-histones complex (DNA60-aH1, Table 5) coincides with the sites found for Con-aH1-0d (Table 2). In contrast to the DNA-histone complex, immunization of mice with MOG (MOG20-aH1) leads not to a decrease but to the increase of sites number against H1 from 16 to 26 sites in comparison to Con-aH1-0d. As a result, the difference in the number of H1 sites hydrolysis by MOG20-aH1 and DNA60-aH1 antibodies is 18 (Tables 3 and 5).
If the spontaneous development of EAE within 60 days results in the appearance of anti-H2A antibodies (Spont-aH2A-60d) that hydrolyze H1 at 25 sites, then immunization of mice with a DNA-histones complex after 60 days leads to a decrease in the number of sites of DNA60-aH2A to 15 (Table 5). In addition, only three hydrolysis sites in the case of Spont-aH2A-60d and DNA60-aH2A antibodies coincide (Table 5). The number of H1 hydrolysis by anti-H2A IgGs at zero time and after immunization of mice with the DNA-histones complex at 60 days (DNA60-aH2A) is comparable - 16 and 15 sites (Tables 2 and 5) but only one site of weak cleavage of them is the same. Of the 4 sites of H1 hydrolysis by IgGs against H2A after mice immunization with MOG (MOG20-aH2A), two coincide with two of 8 sites of H1 degradation by Abs corresponding to Spont-aH2A-60d (Tables 3 and 5).
As a result of the spontaneous development of EAE within 60 days (Spont-aH2B-60d), antibodies against H2B hydrolyze H1 histone at 8 sites, while 60 days after immunization of mice with a DNA-histone complex (DNA60-aH2B) at 12 sites (Table 5). Two sites of hydrolysis of H1 splitting by these antibodies coincide but differ in the efficiency of the hydrolysis of H1 by IgGs against H2B histone. Antibodies against H2B corresponding to the zero time of the experiment (Con-aH2B-0d) hydrolyze H1 only at four sites (Table 2), two of which coincide with those for DNA60-aH2B antibodies. Of the five sites of H1 hydrolysis by antibodies against H2B after treatment of mice with MOG (MOG20-aH2B), three sites are the same as for DNA60-aH2B (Tables 3 and 5). The number of hydrolysis sites in the process of spontaneous development of EAE from 20 (Spont-aH2B-20d) to 60 (Spont-aH2B-60d) days increases from 5 to 12, but there are only two of the same splitting sites for these antibodies (Tables 1 and 5).
It is interesting that the spontaneous development of EAE from zero time (Con-aH3-0d; Table 1) to 60 days of spontaneous EAE (Spont-aH3-60d; Table 1) leads to a decrease in H1 hydrolysis sites by IgGs against H3 histone from 17 to 10, while after immunization of mice with DNA-histones complex, the number of hydrolysis sites after 60 days is 16 (DNA60-aH2B; Table 5). For Spont-aH3-60d and DNA60-aH2B antibodies, only three identical hydrolysis sites are observed. Of the seven sites of hydrolysis of H1 by IgGs after mice treatment with MOG (MOG20-aH3; Table 3), 4 sites are the same as 4 of the 16 sites of H1 splitting of this histone by antibodies against H3 histone (Spont-aH3-60d). During the development of EAE after immunization of mice with a DNA-histones complex from 20 (DNA20-aH3) to 60 (DNA60-aH3) days, there is a change in the set of antibodies against H3 histone, hydrolyzing H1 from 9 to 16 (Tables 3 and 5). Of 7 sites of H1 hydrolysis by antibodies against histone H3, corresponding to the immunization of mice with MOG (MOG20-aH3), 4 sites are the same as 4 of the 16 sites of splitting sites of this histone by DNA60-aH3 antibodies.
If after the spontaneous development of EAE within 60 days, IgGs against H4 hydrolyze H1 at 17 sites (Table1), immunization of mice with a DNA-histones complex leads to a decrease in the number of spitting sites to 6 (Table 5). In addition, there is a development of lymphocytes producing other antibodies (DNA60-aH4), none of the 6 sites of histone H1 hydrolysis by which do not coincide with those for Spont-aH4-60d and only 4 of them are the same as in the case of Con-aH4-0d. At the same time, out of 17 H1 hydrolysis sites for IgGs after immunization with MOG (MOG20-aH4) and 6 sites after mice treatment with the DNA-histone complex, 5 sites coincide (Tables 3 and 5).
Sites of H1 hydrolysis with IgGs against MBP are presented in Table 6.
|
Con-aMBP-0d |
MOG20-aMBP |
DNA20-aMBP |
DNA60-aMBP |
|
5 sites |
3 sites |
2 sites |
8 sites |
|
Y57-K58** |
Y57-K58 |
- |
Y57-K58 |
|
D64-S65** |
- |
D64-S65 |
- |
|
- |
- |
- |
S65-Q66 |
|
- |
- |
- |
K72-R73 |
|
- |
- |
- |
T77-G78 |
|
F106-K107 |
F106-K107 |
F106-K107 |
F106-K107 |
|
K107-K198 |
- |
- |
- |
|
K108-T109 |
K108-T109 |
- |
K108-T109** |
|
- |
- |
- |
K11-E112 |
|
- |
- |
- |
K114-K115 |
|
*The molecular weights of H1 histone splitting products were used for estimation of the cleavage sites based on a set of data from 8-10 spectra.**Major sites of the hydrolysis are shown in red, moderate sites in black, and minor sites in green. Missing cleavage sites are marked with a dash (-). |
|||
Anti-MBP antibodies of 3-month-old mice hydrolyze H1 at only five sites (Table 6). At 20 days, the number of sites after immunization of mice with MOG is decreased to three, and by the DNA-histones complex, to 2. Only 60 days after immunization of mice with DNA-histones complex, the number of hydrolysis sites increases to 8 (Table 6). Thus, anti-MBP antibodies hydrolyze H1 histone at a smaller number of sites than antibodies against five histones.
Discussion
The polyreactivity of complex formation in the case of different Abs is a widespread phenomenon [62-65]. Antibodies' affinity for non-specific antigens is usually significantly lower than for cognate-specific ones, and such antibodies can be traditionally eluted from affinity sorbents by 0.1-0.15 M NaCl [19-23]. Therefore, non-specifically bound Abs were eluted from affinity sorbents using 0.2 M NaCl. IgGs against MBP and five histones, in addition, were additionally passed through alternative affinity sorbents. Finally, the first IgG fractions were obtained against five histones (H1-H4) and MBP containing no alternative IgGs. Then the fraction containing IgGs against five histones was used to prepare antibodies against each of the five individual histones.
It previously showed that polyclonal IgGs from EAE-prone mice used in our study do not contain any canonical proteases [47]. The comparison of H1 splitting sites with IgGs against MBP and five individual histones well supports this conclusion (Figures 7-12, Tables 2-6). Trypsin hydrolyzes various proteins after arginine (R) and lysine (K) residues. The H1 sequence contains 55 sites for potential cleavage of H1 by trypsin after K and 6 sites after R. Chymotrypsin hydrolyzes proteins after F, Y, and W aromatic AAs. However, the number of sites of H1 cleavage by all IgG preparation after K, R, F, and Y residues are relatively small (Figures 7-12 and Tables 2-6).
In contrast to canonical proteases, the sites of H1 hydrolysis by all IgGs are mainly grouped in specific clusters of the histone H1 sequence and mainly occur after neutral AAs: Q, T, G, A, L, and V (Figures 7 -12, Tables 2-6). The specific sites of H1 hydrolysis by all preparations of IgGs against five histones do not correspond to those for trypsin and chymotrypsin. They are not disposed along the entire H1 histone length but are grouped into particular amino acid clusters.
The strong evidence that the IgGs against MBP and five individual histones do not contain even at least noticeable impurities of abzymes against alternative histones follows from the very different number of specific sites of H1 hydrolysis by IgGs against each individual histone and MBP (Figures 7-12, Tables 2-6). Interestingly, the number of H1 hydrolysis sites by different IgG preparations varies from 2 to 26 (Tables 2-6). At the same time, a specific set of hydrolysis sites was found for each IgG preparation, and a very weak coincidence of hydrolysis sites in the case of different IgG preparations (Tables 2-6). These groups of splitting sites differ not only in their location in the protein sequence of histone H1 but also in the same hydrolysis sites found in the case of different IgG preparations vary greatly in the relative efficiency of hydrolysis; major, medium, and minor sites (Figures 7-12, Tables 2-6).
The analysis of which cleavage sites are most often considered major ones in the case of all 29 preparations used is of particular interest. As noted above and can be seen from Tables 2-6, all IgG preparations exhibit various major sites in the hydrolysis of H1 histone. Considering this, it is interesting to understand why at different stages of spontaneous development of EAE and after treatment of mice with MOG and DNA-histones complex, the formation of antibodies hydrolyzing H1 histone at different sites is possible. Therefore, it is useful to take into account some literature data.
First, MS of people is at least two-phase autoimmune pathology [73]. The cascade of reactions at the first inflammatory phase is very complicated, involving many chemokines, cytokines, proteins, enzymes inducing macrophages and other cells producing NO? radicals and osteopathin [73]. The composite coordinated action of B- and T-cells, complement system, mediators of inflammation, and auto-antibodies leads to the formation of demyelination nidi and infringement of axon conductivity. The neurodegenerative stage of MS that appears later is connected directly with the patient’s neural tissue destruction [73]. Therefore, during the analysis of immunological, biochemical, and medicine indices of MS, every current phase of the disease must be considered, including changes in immunoregulation, systemic metabolic changes, and exhaustion of different compensatory and adaptive mechanisms [73]. It should be assumed that in the case of spontaneous and antigen-induced development of mice EAE can proceed in several stages, when at different stages, autoantigens, including histones, and MBP, can form complexes with different proteins, nucleic acids, lipids, polysaccharides, cells, etc.
When analyzing possible reasons of the diversity in the number and type of sites of H1 hydrolysis by Abs against five individual histones, it is important to take into account that the spontaneous development of autoimmune reactions bound with changes in the profile of bone marrow stem cells occurs in at least two stages. At the beginning and acute phase, there is the first, and with the transition to a deep pathology, the second stage of changes in this differentiation profile was observed. Treatment of mice with MOG and the DNA-histone complex strongly alters the pattern of HSCs differentiation compared to its change during the spontaneous development of EAE.
As it is known that the formation of natural Abzs occurs against specific structures of different molecules, imitating, at least to some extent, the transition states of chemical reactions [17-23]. In principle, Abzs could be formed in the case of different antigenic determinants or protein sequences that mimic the transition state of a peptide bond hydrolysis reaction. At various stages of EAE achievement, each of the five histones and their complexes with each other, or with DNA or other different molecules or cells, could represent different fragments of protein sequences that mimic the transition states of hydrolysis of bonds between AAs for the formation of Abzs. Moreover, all these substrate-molecules and their various associates at different stages of EAE achievement could form various complexes with different proteins, lipids, oligosaccharides, etc., which can lead to the formation of specific protein determinants and the formation of some new sequences imitating transition states. This might be one of possible reasons for the formation of very different abzymes hydrolyzing H1 at different sites corresponding to various stages of EAE development.
Conclusion
Here, we have first demonstrated that IgG-abzymes from EAE-prone C57BL/6 mice against five individual histones, and myelin basic protein can form complexes with H1 histone demonstrating polyreactivity in complexation. In addition, all IgGs possess unusual enzymatic cross-reactivity in the hydrolysis of histone H1. IgGs against five individual histones and MBP at the beginning of the experiment (3-mice-old mice) and after spontaneous development of EAE during 60 days differ in the relative number of hydrolysis sites and their type. After mice immunization with MOG or DNA-histones complex, the sites of H1 hydrolysis differ from each other, as well as from those for Abs corresponding to the beginning of the experiment and the spontaneous development of EAE. Overall, depending on the IgG preparation, the number of H1 hydrolysis sites varies from 2 to 26. The treatment of mice with MOG and DNA-histones complex accelerates the EAE evolution and leads to a change in the relative activity of IgGs in H1 hydrolysis. Possible reasons for the catalytic cross-reactivity of antibodies and such strong differences in the number and type of histone H1 cleavage sites were analyzed.
Supplementary Materials
Supplementary data: Supplementary Figures S1, S2, and S3, demonstrate a change of different parameters during the development of EAE in EAE-prone mice.
Author Disclosure Statement
Nothing to disclose.
Acknowledgments
This research was performed with the support of a grant from the Russian Science Foundation, 22-15-00103.
Conflicts of Interest
The authors have no competing financial interests.
References
2. Suwa A. Specificities and clinical significance of autoantibodies directed against histones. Nihon Rinsho Men'eki Gakkai Kaishi= Japanese Journal of Clinical Immunology. 2005 Jun 1;28(3):123-30.
3. Fournel S, Muller S. Anti-nucleosome antibodies and T-cell response in systemic lupus erythematosus. Annales de Medecine Interne. 2002 Dec 1;153(8):513-519.
4. O'connor KC, Bar-Or A, Hafler DA. The neuroimmunology of multiple sclerosis: possible roles of T and B lymphocytes in immunopathogenesis. Journal of Clinical Immunology. 2001 Mar;21:81-92.
5. Archelos JJ, Storch MK, Hartung HP. The role of B cells and autoantibodies in multiple sclerosis. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society. 2000 Jun;47(6):694-706.
6. Hemmer B, Archelos JJ, Hartung HP. New concepts in the immunopathogenesis of multiple sclerosis. Nature Reviews Neuroscience. 2002 Apr 1;3(4):291-301.
7. Croxford AL, Kurschus FC, Waisman A. Mouse models for multiple sclerosis: historical facts and future implications. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2011 Feb 1;1812(2):177-83.
8. Mouse EA. models. Overview and Model Selection Hooke Laboratories. Inc.: Lawrence, MA, USA. 2013.
9. Ikehara S, Kawamura M, Takao F, Inaba M, Yasumizu R, Than S, et al. Organ-specific and systemic autoimmune diseases originate from defects in hematopoietic stem cells. Proceedings of the National Academy of Sciences. 1990 Nov;87(21):8341-4.
10. Aulova KS, Toporkova LB, Lopatnikova JA, Alshevskaya AA, Sedykh SE, Buneva VN, et al. Changes in cell differentiation and proliferation lead to production of abzymes in EAE mice treated with DNA–Histone complexes. Journal of Cellular and Molecular Medicine. 2018 Dec;22(12):5816-32.
11. Aulova KS, Toporkova LB, Lopatnikova JA, Alshevskaya AA, Sennikov SV, Buneva VN, et al. Changes in haematopoietic progenitor colony differentiation and proliferation and the production of different abzymes in EAE mice treated with DNA. Journal of Cellular and Molecular Medicine. 2017 Dec;21(12):3795-809.
12. Doronin VB, Parkhomenko TA, Korablev A, Toporkova LB, Lopatnikova JA, Alshevskaja AA, et al. Changes in different parameters, lymphocyte proliferation and hematopoietic progenitor colony formation in EAE mice treated with myelin oligodendrocyte glycoprotein. Journal of Cellular and Molecular Medicine. 2016 Jan;20(1):81-94.
13. Doronin VB, Korablev A, Toporkova LB, Aulova KS, Buneva VN, Budde T, et al. Changes in several disease parameters including abzymes and hematopoietic progenitor colony formation in brain inflammation and demyelination. J. Neurol. Neurol. Disord. 2017;3:302.
14. Andryushkova AA, Kuznetsova IA, Bineva VN, Toporkova LB, Sakhno LV, Tikhonova MA, et al. Formation of different abzymes in autoimmune‐prone MRL‐lpr/lpr mice is associated with changes in colony formation of haematopoietic progenitors. Journal of Cellular and Molecular Medicine. 2007 May;11(3):531-51.
15. Andryushkova AA, Kuznetsova IA, Orlovskaya IA, Buneva VN, Nevinsky GA. Antibodies with amylase activity from the sera of autoimmune-prone MRL/MpJ-lpr mice. FEBS Letters. 2006 Sep 18;580(21):5089-95.
16. Andryushkova AA, Kuznetsova IA, Orlovskaya IA, Buneva VN, Nevinsky GA. Nucleotide-hydrolyzing antibodies from the sera of autoimmune-prone MRL-lpr/lpr mice. International Immunology. 2009 Aug 1;21(8):935-45.
17. Keinan E. (Ed) Catalytic antibodies. Weinheim, Germany: Wiley-VCH Verlag GmbH and Co, KgaA, 2005, pp. 1-586.
18. Nevinsky GA. Autoimmune processes in multiple sclerosis: Production of harmful catalytic antibodies associated with significant changes in the hematopoietic stem cell differentiation and proliferation. In: Conzalez-Quevedo, A, Ed. Multiple sclerosis. Rijeka, Croatia: InTech; 2016, pp. 100-147.
19. Nevinsky GA, Buneva VN. Natural catalytic antibodies–abzymes. In Keinan E, Ed. Catalytic antibodies. Weinheim: VCH-Wiley Press; 2005, pp. 505–569.
20. Nevinsky GA. Natural catalytic antibodies in norm and in Autoimmune Diseases. In: Brenner KJ, Ed. Autoimmune Diseases: Symptoms, Diagnosis and Treatment. New York, NY, USA: Nova Science Publishers Inc; 2010, pp. 1-107.
21. Nevinsky GA. Natural catalytic antibodies in norm and in HIV-infected patients. In: Kasenga, FH, Ed. Understanding HIV/AIDS Management and Care—Pandemic Approaches the 21st Century. Rijeka, Croatia: InTech; 2011, pp. 151-192.
22. Nevinsky GA. Catalytic antibodies in norm and systemic lupus erythematosus. In: Khan WA, Ed. Lupus. Rijeka, Croatia: InTech; 2017 May 31:41-101.
23. Nevinsky GA. The extreme diversity of autoantibodies and abzymes against different antigens in patients with various autoimmune diseases. In: Advances in Medicine and Biology. New York, NY, USA: Nova Science Publishers Inc; 2021, 184. pp. 1-130.
24. Savel'ev AN, Eneyskaya EV, Shabalin KA, Filatov MV, Neustroev KN. Autoantibodies with amylolytic activity. Protein and Peptide Letters. 1999 Jun 1;6:179-81.
25. Li L, Paul S, Tyutyulkova S, Kazatchkine MD, Kaveri S. Catalytic activity of anti-thyroglobulin antibodies. Journal of Immunology (Baltimore, Md.: 1950). 1995 Apr 1;154(7):3328-32.
26. Kalaga R, Li L, O'Dell JR, Paul S. Unexpected presence of polyreactive catalytic antibodies in IgG from unimmunized donors and decreased levels in rheumatoid arthritis. Journal of Immunology (Baltimore, Md.: 1950). 1995 Sep 1;155(5):2695-702.
27. Paul S, Volle DJ, Beach CM, Johnson DR, Powell MJ, Massey RJ. Catalytic hydrolysis of vasoactive intestinal peptide by human autoantibody. Science. 1989 Jun 9;244(4909):1158-62.
28. Paul S, Planque SA, Nishiyama Y, Hanson CV, Massey RJ. Nature and nurture of catalytic antibodies. Naturally Occurring Antibodies (NAbs). 2012:56-75.
29. Planque SA, Nishiyama Y, Hara M, Sonoda S, Murphy SK, Watanabe K, et al. Physiological IgM class catalytic antibodies selective for transthyretin amyloid. Journal of Biological Chemistry. 2014 May 9;289(19):13243-58.
30. Baranovskii AG, Kanyshkova TG, Mogelnitskii AS, Naumov VA, Buneva VN, Gusev EI, et al. Polyclonal antibodies from blood and cerebrospinal fluid of patients with multiple sclerosis effectively hydrolyze DNA and RNA. Biochemistry. Biokhimiia. 1998 Nov 1;63(11):1239-48.
31. Baranovskii AG, Ershova NA, Buneva VN, Tat'yana GK, Mogelnitskii AS, Doronin BM, et al. Catalytic heterogeneity of polyclonal DNA-hydrolyzing antibodies from the sera of patients with multiple sclerosis. Immunology Letters. 2001 Apr 2;76(3):163-7.
32. Vlassov A, Florentz C, Helm M, Naumov V, Buneva V, Nevinsky G, et al. Characterization and selectivity of catalytic antibodies from human serum with RNase activity. Nucleic Acids Research. 1998 Dec 1;26(23):5243-50.
33. Polosukhina DI, Kanyshkova TG, Doronin BM, Tyshkevich OB, Buneva VN, Boiko AN, et al. Hydrolysis of myelin basic protein by polyclonal catalytic IgGs from the sera of patients with multiple sclerosis. Journal of Cellular and Molecular Medicine. 2004 Jul;8(3):359-68.
34. Polosukhina DI, Buneva VN, Doronin BM, Tyshkevich OB, Boiko AN, Gusev EI, et al. Hydrolysis of myelin basic protein by IgM and IgA antibodies from the sera of patients with multiple sclerosis. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research. 2005 Jul 25;11(8):BR266-72.
35. Dar’ya IP, Tat’yana GK, Doronin BM, Tyshkevich OB, Buneva VN, Boiko AN, et al. Metal-dependent hydrolysis of myelin basic protein by IgGs from the sera of patients with multiple sclerosis. Immunology Letters. 2006 Feb 28;103(1):75-81.
36. Bezuglova AM, Konenkova LP, Doronin BM, Buneva VN, Nevinsky GA. Affinity and catalytic heterogeneity and metal‐dependence of polyclonal myelin basic protein‐hydrolyzing IgGs from sera of patients with systemic lupus erythematosus. Journal of Molecular Recognition. 2011 Nov;24(6):960-74.
37. Baranova SV, Mikheeva EV, Buneva VN, Nevinsky GA. Antibodies from the sera of multiple sclerosis patients efficiently hydrolyze five histones. Biomolecules. 2019 Nov 15;9(11):741.
38. Parkhomenko TA, Doronin VB, Castellazzi M, Padroni M, Pastore M, Buneva VN, et al. Comparison of DNA-hydrolyzing antibodies from the cerebrospinal fluid and serum of patients with Multiple Sclerosis. PLoS One. 2014 Apr 15;9(4):e93001.
39. Doronin VB, Parkhomenko TA, Castellazzi M, Padroni M, Pastore M, Buneva VN, et al. Comparison of antibodies hydrolyzing myelin basic protein from the cerebrospinal fluid and serum of patients with multiple sclerosis. PLoS One. 2014 Sep 29;9(9):e107807.
40. Doronin VB, Parkhomenko TA, Castellazzi M, Cesnik E, Buneva VN, Granieri E, et al. Comparison of antibodies with amylase activity from cerebrospinal fluid and serum of patients with multiple sclerosis. PLoS One. 2016 May 19;11(5):e0154688.
41. Baranova SV, Buneva VN, Nevinsky GA. Antibodies from the sera of HIV‐infected patients efficiently hydrolyze all human histones. Journal of Molecular Recognition. 2016 Aug;29(8):346-62.
42. Baranova SV, Dmitrienok PS, Ivanisenko NV, Buneva VN, Nevinsky GA. Antibodies to H1 histone from the sera of HIV‐infected patients recognize and catalyze site‐specific degradation of this histone. Journal of Molecular Recognition. 2017 Mar;30(3):e2588.
43. Baranova SV, Dmitrienok PS, Ivanisenko NV, Buneva VN, Nevinsky GA. Antibodies to H2a and H2b histones from the sera of HIV-infected patients catalyze site-specific degradation of these histones. Molecular Biosystems. 2017;13(6):1090-101.
44. Baranova SV, Dmitrenok PS, Zubkova AD, Ivanisenko NV, Odintsova ES, Buneva VN, et al. Antibodies against H3 and H4 histones from the sera of HIV‐infected patients catalyze site‐specific degradation of these histones. Journal of Molecular Recognition. 2018 Jul;31(7):e2703.
45. Baranova SV, Dmitrienok PS, Buneva VN, Nevinsky GA. Autoantibodies in HIV‐infected patients: cross site‐specific hydrolysis of H1 histone and myelin basic protein. Biofactors. 2019 Mar;45(2):211-22.
46. Baranova SV, Dmitrienok PS, Buneva VN, Nevinsky GA. HIV-infected patients: Cross site-specific hydrolysis of H2a and H2b histones and myelin basic protein with antibodies against these three proteins. Biomolecules. 2020 Oct 30;10(11):1501.
47. Urusov AE, Aulova KS, Dmitrenok PS, Buneva VN, Nevinsky GA. Experimental autoimmune encephalomyelitis of mice: Enzymatic cross site-specific hydrolysis of H4 histone by iggs against histones and myelin basic protein. International Journal of Molecular Sciences. 2022 Aug 16;23(16):9182.
48. Sinohara H, Matsuura K. Does catalytic activity of Bence-Jones proteins contribute to the pathogenesis of multiple myeloma?. Applied Biochemistry and Biotechnology. 2000 Jan;83:85-92.
49. Kozyr AV. Autoantibodies to nuclear antigens: correlation between cytotoxicity and DNA-hydrolyzing activity. Appl. Biochem. Biotechnol.. 1998;75:45-61.
50. Nevinsky GA, Buneva VN. Catalytic antibodies in healthy humans and patients with autoimmune and viral diseases. Journal of Cellular and Molecular Medicine. 2003 Jul;7(3):265-76.
51. Ternynck T, Falanga PB, Unterkirsche C, Gregoire J, da Silva LP, Avrameas S. Induction of high levels of IgG autoantibodies in mice infected with Plasmodium chabaudi. International Immunology. 1991 Jan 1;3(1):29-37.
52. Hentati B, Sato MN, Payelle‐Brogard B, Avrameas S, Ternynck T. Beneficial effect of polyclonal immunoglobulins from malaria‐infected BALB/c mice on the lupus‐like syndrome of (NZB× NZW) F1 mice. European Journal of Immunology. 1994 Jan;24(1):8-15.
53. Matsiota-Bernard P, Mahana W, Avrameas S, Nauciel C. Specific and natural antibody production during Salmonella typhimurium infection in genetically susceptible and resistant mice. Immunology. 1993 Jul;79(3):375-80.
54. Barzilai O, Ram M, Shoenfeld Y. Viral infection can induce the production of autoantibodies. Current Opinion in Rheumatology. 2007 Nov 1;19(6):636-43.
55. Libbey JE, Cusick MF, Fujinami RS. Role of pathogens in multiple sclerosis. International Reviews of Immunology. 2014 Jul 4;33(4):266-83.
56. Andersen O, Lygner PE, Bergström T, Andersson M, Vablne A. Viral infections trigger multiple sclerosis relapses: a prospective seroepidemiological study. Journal of Neurology. 1993 Jul;240:417-22.
57. Nishi Y. Evolution of catalytic antibody repertoire in autoimmune mice. Journal of Immunological Methods. 2002 Nov 1;269(1-2):213-33.
58. Tawfik DS, Chap R, Green BS, Sela M, Eshhar Z. Unexpectedly high occurrence of catalytic antibodies in MRL/lpr and SJL mice immunized with a transition-state analog: is there a linkage to autoimmunity?. Proceedings of the National Academy of Sciences. 1995 Mar 14;92(6):2145-9.
59. NEVINSKY GA. Structural, thermodynamic, and kinetic basis of DNA-and RNA-dependent enzymes functioning. Important role of weak nonspecific additive interactions between enzymes and long nucleic acids for their recognition and transformation. In: Uversky VN, Ed. Protein Structures: Kaleidoscope of Structural Properties and Functions. Kerala, India: Research Signpost; 2003, pp. 133-222
60. Nevinsky GA. Structural, thermodynamic, and kinetic basis for the activities of some nucleic acid repair enzymes. Journal of Molecular Recognition. 2011 Jul;24(4):656-77.
61. Nevinsky GA. How Enzymes, Proteins, and Antibodies Recognize Extended DNAs; General Regularities. International Journal of Molecular Sciences. 2021 Jan 29;22(3):1369.
62. Zhou ZH, Tzioufas AG, Notkins AL. Properties and function of polyreactive antibodies and polyreactive antigen-binding B cells. Journal of Autoimmunity. 2007 Dec 1;29(4):219-28.
63. James LC, Roversi P, Tawfik DS. Antibody multispecificity mediated by conformational diversity. Science. 2003 Feb 28;299(5611):1362-7.
64. James LC, Tawfik DS. Conformational diversity and protein evolution–a 60-year-old hypothesis revisited. Trends in Biochemical Sciences. 2003 Jul 1;28(7):361-8.
65. James LC, Tawfik DS. The specificity of cross‐reactivity: promiscuous antibody binding involves specific hydrogen bonds rather than nonspecific hydrophobic stickiness. Protein Science. 2003 Oct;12(10):2183-93.
66. Khaitov RM, Ignatieva GA, Sidorovich IG. Immunology. Medicine: Moscow, Russia, 2000, pp. 1-432.
67. Timofeeva AM, Buneva VN, Nevinsky GA. Systemic lupus erythematosus: Molecular cloning and analysis of 22 individual recombinant monoclonal kappa light chains specifically hydrolyzing human myelin basic protein. Journal of Molecular Recognition. 2015 Oct;28(10):614-27.
68. Timofeeva AM, Buneva VN, Nevinsky GA. Systemic lupus erythematosus: Molecular cloning and analysis of recombinant monoclonal kappa light chain NGTA1-Me-pro with two metalloprotease active centers. Molecular BioSystems. 2016;12(12):3556-66.
69. Bezuglova AM, Buneva VN, Nevinsky GA. Systemic lupus erythematosus: Monoclonal light chains of immunoglobulins against myelin basic protein, have proteolytic and DNase activity. Russ. J. Immunol. 2011;5:215-25.
70. Timofeeva AM, Ivanisenko NV, Buneva VN, Nevinsky GA. Systemic lupus erythematosus: molecular cloning and analysis of recombinant monoclonal kappa light chain NGTA2-Me-pro-ChTr possessing two different activities—trypsin-like and metalloprotease. International Immunology. 2015 Dec 1;27(12):633-45.
71. Timofeeva AM, Nevinsky GA. Systemic lupus erythematosus: Possible localization of trypsin‐like and metalloprotease active centers in the protein sequence of the monoclonal light chain (NGTA2‐Me‐pro‐Tr). Biotechnology and Applied Biochemistry. 2020 Nov;67(6):946-59.
72. Timofeeva AM, Buneva VN, Nevinsky GA. Systemic Lupus Erythematosus: Localization of DNase, Trypsin-Like, and Metalloprotease Active Centers in the Protein Sequence of NGTA3-Pro-Dnase Monoclonal Light Chain of Human Antibodies. J. Mol. Biol. Mol. Imaging. 2020;6:1031.
73. Steinman L. Multiple sclerosis: a two-stage disease. Nature Immunology. 2001 Sep 1;2(9):762-4.