Abstract
Optimum nutrition is the key for growth and neurodevelopment of the vulnerable preterm neonates, yet immaturity of the gut and risk of necrotizing enterocolitis in this subgroup of neonates create hesitancy regarding aggressive feeding. Feeding practices in the preterm babies are heterogeneous across various neonatal intensive care units. Many neonatal units still differ in terms of feeding intervals, volume of feed initiation and slow versus fast advancement of feeds.
The recommendations are strong for the type of feeding of preterm neonates, however the initiation and increment of feeds is still debatable, especially in extreme preterm neonates. The current review brings forth 9 key recommendations based on the recent evidence in preterm feeding.
Keywords
Preterm, Feeding, Enteral feeding, Feed intolerance, Very low birth weight
Introduction
Prematurity leaves the neonate deprived of the period of substantial intrauterine nutrition, which happens in the last trimester. With the cutting of umbilical cord at birth, these neonates undergo negative nitrogen balance. Studies [1] show that they are unable to catch up with cumulative deficit in amino acid and energy intake and land up with extra-uterine growth retardation (EUGR), hence making preterm birth a nutritional emergency. The incidence of EUGR in preterm neonates has been reported to range from as high as 86% [2] to 15% [3] in some units. Literature [4] shows that time to reach full enteral feeds is one of the factors responsible for extra-uterine growth restriction. Early Nutrition also holds the key to brain growth and neurodevelopment [5]. The type of feeding to be initiated has stronger evidence-based recommendations, with the European Society for Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) [6], American Academy of Pediatrics (AAP) and Milan EMBA/ESPGHAN/AAP recommending mother’s own milk (MOM) as the first choice in the feeding of preterm infants. However, the choice of alternative feeds in case of non-availability of mothers’ own milk in the initial 48 hours after birth remains a challenge as does practice variations in initiation and increment of feeds.
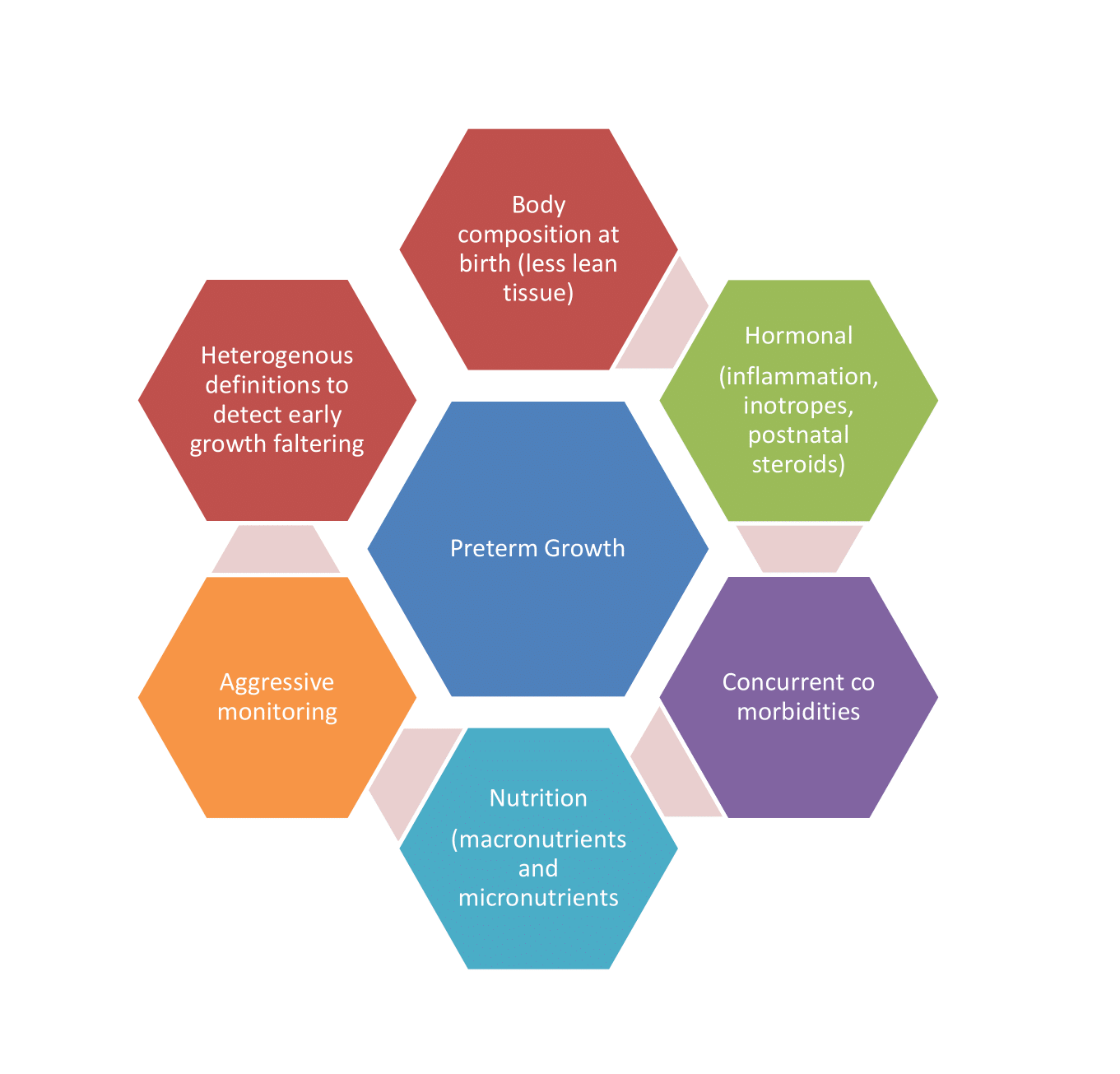
With the existing dilemmas regarding the feeding of preterm neonates (Figure 1), this review aims to appraise about the most recent evidence in context to: (i) Initiation of feeds in very low birth weight (VLBW) between 1000-1499 grams (ii) Initiation of feeds in extremely low birth weight (ELBW) less than 1000 grams, very preterm less than 32 weeks and extreme preterm less than 28 weeks gestation, (iii) Feeding of small for gestation age (SGA) (birth weight less than 10th centile as defined by WHO) [7] (iv) Advancement of feeds (gradual versus fast) (v) Maximal feeds to be given in preterm neonates (vi) Feeding interval in preterm neonates (vii) Monitoring for feed intolerance and recommendations for transitional feeding and fortification of feeds in preterm neonates.
Figure 1: Dilemmas regarding the feeding of preterm neonates.
Initiation of Enteral Feeds in VLBW Neonates
Cochrane review in 2013 [8] incorporating nine trials and 754 very preterm or very low birth weight babies compared early trophic feeding (milk volumes up to 24 ml/kg/day introduced before 96 hours postnatal age and continued until at least one week after birth) with enteral fasting during same duration in very preterm or VLBW neonates. They found no adverse effects of early trophic feeding on all-cause mortality, necrotizing enterocolitis (RR 1.07, 95%CI 0.67-1.70), feed tolerance, growth rates or late onset sepsis.
Further, meta-analysis [9] of 964 VLBW infants from nine trials found that delayed introduction of feeds (5-7 days after birth) versus early introduction (within 4 days after birth) resulted in no significant effect on the risk of all-cause mortality or NEC.
Comparison of enteral fasting versus trophic feeding and delayed versus early introduction of trophic feeds showed relative risk (95%CI) to be 1.51 (0.93-2.44) and 1.26 (0.78-2.01) for all-cause mortality; 1.06 (0.67-1.69) and 0.92 (0.64-1.34) for risk of necrotizing enterocolitis, respectively. A study by the SIFT (Speed of Increasing Milk Feeds Trial) [10] group advocated that progressive enteral feeding can be introduced before 4 days of life, rather they recommended very early introduction and advancement of enteral feeds (within 24 to 48 hours after birth) at more than 24 ml/kg/day increments in very preterm or VLBW infants without increasing risk of NEC.
Further evidence emerged and built up the case for full enteral feeding in VLBW neonates [11]. Feasibility and safety of starting full exclusive enteral feeds early within 24 hours of life and in fact right from birth in VLBW neonates was established [12].
Randomized unblinded control trial on 180 stable VLBW neonates by Nangia et al. [13] in 2019 weighed early total enteral feeding (ETEF) against conventional enteral feeding (CEF: minimal enteral nutrition 20 mL/kg along with iv supplementation of rest of the requirement). They concluded that ETEF in stable VLBW infants resulted in earlier attainment of full feeds (6.5 ± 1.5 vs. 10.1 ± 4.1 days postnatal age; p<0.001) and shorter duration of hospital stay without any increased risk of feed intolerance or NEC. 30% of the neonates were SGA in this trial.
Recent Cochrane review published in 2020 [14] comprising of 526 neonates between 1000 to 1500 grams, consisting of six trials where babies started on full enteral feeds at 80 ml/kg/day were compared to the other group started on trophic feeds plus intravenous fluids and increment by 20-30 ml/kg/day in both groups till they reached volumes of 150-180 ml/kg/day. Meta-analysis by Salas et al. [15] showed no evidence of effect on mortality or necrotizing enterocolitis (RR 0.98, 95% CI 0.38 to 2.54).
Recommendation 1: Start exclusive full enteral feeds right at birth directly in stable very low birth weight VLBW neonates (1000-1500 grams).
Initiation of Feeding in Extreme Preterm and ELBW Neonates
While feeding is streamlined in most VLBW neonates, still robust evidence is not available for extremely low birth weight and extreme preterm neonates. In all above trials, very few participants were extremely preterm (<28 weeks) or extremely low birth weight (<1000 grams) or growth restricted and none had absented or reversal of Doppler flow. An observational study [13] on <1000 grams extreme preterm neonates evaluated short (<3 days) trophic versus long trophic feeding and found the former reached full feeds earlier but there was no evidence on NEC. A randomized trial by Modi et al. [16] for aggressive initiation and advancement at 30 ml/kg for 750-1000 grams and at 40 ml/kg for 1000-1250 grams versus conventional feeding showed feasibility and are well tolerated without increasing NEC or late onset sepsis but the study was underpowered to detect the primary outcome of reduction in all-cause mortality.
Recommendation 2: Trophic feeding should be started in ELBW or extreme preterm neonates within 24 hours of life at 24 ml/kg/day. Rest of the requirement is to be met by intravenous fluids.
SGA with Absent or Reversal of End Diastolic Flow (AREDF)
Absent or reversal of end diastolic flow in the umbilical artery is thought to cause splanchnic ischemia and feed intolerance in these neonates. However, the available evidence with ADEPT (Abnormal Doppler enteral Prescription Trial) [17] involving 54 centers in UK, comprising of 444 VLBW babies with gestation less than 35 weeks, who were randomized to start feeds in 2 days versus 6 days revealed that full feeds were achieved later (median 28 days) in infants <29 weeks as compared to 19 days in neonates with gestation ≥ 29 weeks (HR 0.35, 95% CI 0.3 to 0.5). The incidence of necrotizing enterocolitis was also higher in this group; 32/83 (39%) compared to 32/312 (10%) in those ≥ 29 weeks (RR 3.7, 95% CI 2.4 to 5.7).
More recently, in a Randomized trial [18], neonates with AREDF (ten extreme preterm and 21 very preterm), were randomized to each early initiation of MEN feeding (within 12-18 hours) and late feeding (at 120-148 hours). They reported faster attainment of sufficient feeds in the early feeding arm of both the stratified groups and there was no difference in the incidence of NEC or feed intolerance. Another randomized trial on SGA babies [19] found that infants included in the very early feeding regimen (within 24 hours) achieved full enteral feeding sooner than controls (98 ± 80-157 vs. 172 ± 123-261 hours of age, respectively; p=0.004) and were discharged home earlier (p=0.04) with no necrotizing enterocolitis (NEC) in either study groups. Besides the above large multi-centric randomized trial, Cochrane review [20] on delayed initiation of enteral feeds (more than 4 days) did not reduce the incidence of NEC or feed intolerance; in fact, the delayed initiation of enteral feeds did show a trend toward more invasive infections.
Recommendation 3: Trophic Feeds can be initiated within 12-24 hours of life in SGA neonates with AREDF, if less than 35 weeks gestation, with monitoring for feed intolerance.
Advancement of Feeds in Preterm Neonates
In very preterm or VLBW neonates, slow advancement of feeds (15-20 ml/kg) as compared to rapid (30-40 ml/kg) has shown longer time to reach full feeds with mean difference (MD) 2-5 days or to regain birth weight (MD 2-6 days) and has favored rapid advancement [19]. Similar results were found by SIFT group [10] for slow versus rapid feed increments, RR (95%CI) were 1.41 (0.81-2.47] for all-cause mortality and 0.97 (0.54-1.74) for risk of NEC.
Meta-analysis [21] of 3753 infants comprising mostly of stable very preterm infants with birth weight appropriate for gestation, one-third (1200) extremely preterm or ELBW (750-1000 grams) and one-fifth (n=600) were SGA, growth-restricted neonates, or compromised in utero with absent or reversed end-diastolic flow velocity (AREDFV, n=440). Trials typically defined slow advancement as daily increments of 15 to 20 mL/kg, and faster advancement as daily increments of 30 to 40 mL/kg. Meta-analyses [22] did not show effects on risk of all-cause mortality (RR 1.15, 95% CI 0.93 to 1.42) or NEC (RR 1.07, 95% CI 0.83 to 1.39). Slow feed advancement delayed the achievement of full enteral nutrition and brought out borderline increased risk of invasive infection (RR 1.15, 95% CI 1.00 to 1.32). Subgroup analyses of extremely preterm or ELBW infants, or of SGA or growth-restricted or growth-compromised infants also did not show any increase in mortality or NEC [22] nor in very preterm or VLBW [23].
Recommendation 4: Increase feed volume by 30-40 ml/kg/day in very low birth weight neonates (1000-1500 grams), extreme low birth weight (750-1000 grams), very preterm, extreme preterm, SGA and babies with AREDF.
Maximum Volume of Feeds
ESPHGAN 2010 recommendations [24] for preterm neonates are as follows: energy: 110–135 kcal/kg/d, protein: 4.0–4.5 g/kg/d (<1 kg) and 3.5–4.0 g/kg/d (1-1.8 kg), carbohydrate: 11.6–13.2 g/kg/d, fat: 4.8–6.6 g/kg/d). Recommended volume range is 135-200 ml/kg/day [24]. The volume of feeds which should be prescribed also depends on whether feeds have been fortified or unfortified.
Also, for best neurodevelopmental outcomes as per the ESPHGAN recommendations [24], weight gain of 18 grams/kg/d and occipito-frontal circumference (OFC) of 0.9 cm/week is desired. If adequate weight gain is not documented, then fortification is recommended, so that in addition to calories which do increase the weight of the neonate, proteins and other micronutrients which play a crucial role in neurodevelopmental outcome, are being provided as per Recommended Daily Allowances (RDA) for a preterm neonate.
To achieve the recommended 4 g/kg/day of protein in ELBW, babies need to be fed 290-340 ml/kg/day of unfortified breast milk or 210 ml/kg/day of HMF fortified breast milk (4 g/100 ml expressed breast milk) or 170 ml/kg/day to 190 ml/kg/day of preterm formula.
Cochrane update review [25] including 3 randomized trials comparing high volume feeds (defined as ≥ 180 mL/kg/day of fortified human milk or preterm formula or ≥ 200 mL/kg/day of unfortified human milk or term formula) showed improved weight gain during hospital stay mean difference in g/kg/day (95%CI) 2.58 (1.41-3.76) with fortified and 6.2 (2.71 to 9.69) with unfortified milk. However, evidence for other outcomes (NEC, feed intolerance, hypoglycemia, all-cause mortality) was inconclusive [25].
In case of evolving BPD at around 3 weeks of life, if baby is on respiratory support, fluids are to be restricted at 160 ml/kg/day. One study [26] showed that infants with CLD/BPD who were fed formula enriched with protein and minerals calorie >135/kg/day as compared to standard 98-135 kcal/kg/day showed improved growth parameters. 60 neonates with persistent oxygen requirement at 28 days of life were randomized to either 180 mL/kg/day of standard formula or 145 mL/kg/day of concentrated formula did not provide data regarding mortality or oxygen need at 36 weeks PMA and no effects were found on any secondary outcomes like weight gain [26].
Recommendation 5: Maximal fluid volume can be upto-200/kg/day and if there is no adequate weight gain or evolving BPD, restrict the volume to 160 ml/kg/day and use fortified human milk.
Feeding Interval in Preterm Neonates
Feeding a preterm neonate takes up to 25% of the time of nursing staff in neonatal intensive care units. Preterm neonates more than 1250 grams can be safely fed at three hourly feeding intervals with no difference in time to reach full feeds and without increasing risk of NEC, hypoglycemia, or feed intolerance whereas lower weight babies should be fed two hourly [27]. A recent systematic review and meta-analysis [28] on feeding interval in preterm neonates which included 7 studies (4 randomized trials and 3 observational trials) found no significant differences in the outcomes of time to reach full enteral feeding, necrotizing enterocolitis, feed intolerance, and hypoglycemia. But conclusive evidence for the same outcomes was lacking in ELBW neonates [27]. Another Cochrane review [29] found similar results for neonates above 1000 grams for all of these outcomes, the only difference was that they found ELBW neonates fed at short (1-2 hourly) feeding intervals had lesser number of days to reach full enteral feeds as compared to long intervals (3-4 hourly).
Recommendation 6: Feeding 3-hourly is comparable to 2-hourly feeding in preterm infants. However, ELBW neonates reach full enteral feeds earlier when fed 2-hourly compared with 3-hourly.
Monitoring for Feed Intolerance, How to Ensure Transition of Enteral Feeds to Breastfeeds and Recommendations for Fortification of Feeds
Feed intolerance
Presence of two or more of following classify as feed intolerance: pre-feed aspirate >50% of feed volume to be checked after 3 feeds, bilious or altered or fresh bleed in pre-feed aspirate, >one vomitus with yellow or green color or altered blood, abdominal girth more than 2 cm increased over baseline in 24 hours. If feed intolerance is present, then withhold feeds and evaluate for NEC/sepsis. If pre-feed aspirate is <25%, reefed the residual and subtract the same from the next feed volume. If >25-50% and milky, feeds should be interrupted for 6 hours and then reassess.
Few studies reported the benefit of re-feeding the aspirates, as they may replace partially digested milk and gastrointestinal secretions that are essential for gastrointestinal maturation. There is insufficient evidence to support the decision [30].
There is no role of routine monitoring of abdominal girth or gastric residuals in absence of other signs of feed intolerance [31]. Routine monitoring of gastric residual [32] may have little or no effect on the incidence of NEC (risk ratio (RR) 3.07, 95%CI 0.50 to 18.77; participants=141, 2 studies). Routine monitoring increases the risk of feed interruption episodes (RR 2.07, 95% CI 1.39 to 3.07; participants=141; studies=2; low-quality evidence); the number needed to treat for an additional harmful outcome (NNTH) was 3 (95% CI 2 to 6).
Recommendation 7: Routine abdominal girth measurements may increase the risk of feed interruptions. Pre feed aspirates are to be measured only in the presence of abdominal distension.
Transition of feeding
Neonates above 34 weeks gestation have good coordination sucking and swallowing and can be breast-fed directly. Neonates who are unable to take direct breast feeds should be fed with a cup. Meta-analysis [33] of five trials showed better breastfeeding rates at three months (three studies) (typical RR 0.83, 95% CI 0.71 to 0.97) with cup feeds as compared to bottle feeding, though there was no difference in weight gain.
VLBW neonates who are not able to accept cup/spoon/paladai feeds should be given orogastric or nasogastric tube feeding. Position of feeding tube (NG/OG) after placement and before commencement of first feed is recommended in LBW infants. Intragastric route of tube feeding is preferred over trans-pyloric route in preterm infants [34].
Non-nutritive sucking (NNS) is oro-motor sucking at emptied maternal breast or pacifier to and should be offered to preterm neonates less than 32 weeks gestation till they are on tube feeds and should be started as early as 30 weeks of gestation. Cochrane review [35] in 2016 including 12 eligible trials, 746 preterm neonates demonstrated benefits of NNS on transition from gavage to full oral feeding (MD −5.51 days, 95% CI −8.20 to −2.82; N=87), transition from start of oral feeding to full oral feeding (MD −2.15 days, 95% CI −3.12 to −1.17; N=100), and the length of hospital stay (MD −4.59 days, 95% CI −8.07 to −1.11; N=501). Meta-analysis revealed no significant effect of NNS on weight gain. One study [35] found that the NNS group had a significantly shorter intestinal transit time during gavage feeding compared to the control group (MD −10.50 h, 95% CI −13.74 to −7.26; N=30].
Recommendation 8: VLBW neonates who are unable to accept cup/paladai feeds are to be given feeds through nasogastric/orogastric route. Breastfeeding rates are better with cup feeds compared to bottle feeds. For transition from cup feeds to breastfeeds, there is limited evidence to recommend nonnutritive sucking in preterm neonates.
Fortification
Human milk fortification is now recommended [36] in all neonates less than 1800 grams once they reach 50-80 ml/kg/day of feeds. However, in in low-income settings, it is advised to start in baby not achieving adequate weight gain at full volume feeds of more than 200ml/kg/day.
ESPHGAN [24] recommends following nutrient requirements for preterm infants (all per kg per day): Energy: 110-135kcal, protein 3.5-4.5g, fat 4.8-6.6 g, carbohydrates 11.6-13.2 g, calcium 120-140mg, Phosphate 60-90 mg [37], vitamin D 800-1000IU till six months of age [38] and Iron 2-3 mg per kg per day till 6-12 months of age [39]. Preterm need fortified human milk for adequate weight gain and prevent extra-uterine growth restriction defined by Goldberg et al [40].
Early start of fortification [41] offers no added advantage of starting early at 20-40 ml/kg/day of feeds, though well tolerated. Cochrane review [42] with 18 trials, 1456 preterm infants show multi-nutrient fortification of human milk increases in-hospital rate of weight gain with no increased risk of NEC.
Standard fortification assumes uniform macronutrient composition of breast milk, individualized fortification can be adjusted which titrates protein supplementation to achieve target blood urea nitrogen (BUN) to 9-16mg/dl, measured twice weekly [43]. Targeted fortification depends on the breast milk analysis and individual component supplementation [44].
Fortification of post-discharge feeding: Most neonates are discharged at 35-36 weeks post-conception age (PCA) and can exclusively be breastfeed if weight has reached 1800 grams. ESPHGAN [24] recommends that babies who are AGA at their PCA, should be breast fed and if suboptimal weight for PCA, then fortified human milk should be given up to least 40 weeks and when SGA up to 52 weeks. If baby is on preterm formula milk, it may be used till baby reaches 3.5-4 kg weight and then limit use to some feeds. Meta-analysis [45,46] of 11 trials compared feeding infants with ‘post discharge formula’ (energy density about 74 kcal/100 mL) versus standard term formula (about 67 kcal/100 mL), reported no evidence of effects on growth parameters up to 12 to 18 months post term.
Fortification of post-discharge feeding: Most neonates are discharged at 35-36 weeks post-conception age (PCA) and can exclusively be breastfeed if weight has reached 1800 grams. ESPHGAN [24] recommends that babies who are AGA at their PCA, should be breast fed and if suboptimal weight for PCA, then fortified human milk should be given up to least 40 weeks and when SGA up to 52 weeks. If baby is on preterm formula milk, it may be used till baby reaches 3.5-4 kg weight and then limit use to some feeds. Meta-analysis [45,46] of 11 trials compared feeding infants with ‘post discharge formula’ (energy density about 74 kcal/100 mL) versus standard term formula (about 67 kcal/100 mL), reported no evidence of effects on growth parameters up to 12 to 18 months post term.
Recommendation 9: Despite intake 200 ml/kg/day of expressed breast milk, if baby weighing less than 1800 grams is not gaining weight adequately (at 18g/kg/day), fortification with human milk fortifier is recommended. Routine fortification of breast milk and post-discharge formula are not recommended after discharge of the neonate unless baby has suboptimal weight for the post-conception age.
Conclusion
This review has tried to comprehensively address the common daily challenges faced while enterally feeding preterm neonates. We summarize the recommendations as given below for uniformity in feeding practices (Table 1).
|
S.No. |
Feeding strategy |
Recommendation |
Level of evidence |
|
1 |
Initiation of enteral feeding in VLBW neonates |
Start exclusive full enteral feeds right at birth directly in stable very low birth weight VLBW neonates (1000-1500 grams). |
Level 1a |
|
2 |
Initiation of enteral feeding in ELBW neonates |
Trophic feeding should be started in ELBW or extreme preterm neonates within 24 hours of life at 24 ml/kg/day. Rest of the requirement is to be met by intravenous fluids. |
Level 2b |
|
3 |
Initiation of feeds in SGA with AEDF |
Trophic Feeds can be initiated within 12-24 hours of life in SGA neonates with AREDF, if less than 35 weeks gestation, with monitoring for feed intolerance. |
Level 2b |
|
4 |
Advancement of feeds in preterm neonate |
Increase feed volume by 30-40 ml/kg/day in very low birth weight neonates (1000-1500 grams), extreme low birth weight (750-1000 grams), very preterm, extreme preterm, SGA and babies with AREDF. |
Level 1b |
|
5 |
Maximal volume of feeds in a preterm neonate |
Maximal fluid volume can be upto-200 ml/kg/day and if there is no adequate weight gain or evolving BPD, restrict the volume to 160 ml/kg/day and use fortified human milk. |
Level 1b |
|
6 |
2 hourly versus 3 hourly feeding intervals in a preterm neonate |
Feeding 3-hourly is comparable to 2-hourly feeding in preterm infants. However, ELBW neonates reach full enteral feeds earlier when fed 2-hourly compared with 3-hourly. |
Level 1b |
|
7 |
Monitoring for feed intolerance |
Routine abdominal girth measurements may increase the risk of feed interruptions. Pre feed aspirates are to be measured only in the presence of abdominal distension. |
Level 2b |
|
8 |
Transitional feeding from cup to breastfeeding and role of nonnutritive sucking |
VLBW neonates who are unable to accept cup/paladai feeds are to be given feeds through nasogastric/orogastric route. Breastfeeding rates are better with cup feeds compared to bottle feeds. For transition from cup feeds to breastfeeds, there is limited evidence to recommend nonnutritive sucking in preterm neonates. |
Level 1a |
|
9 |
Role of fortification of feeds during hospital stay and in post discharge feeds. |
Despite intake 200 ml/kg/day of expressed breast milk, if baby weighing less than 1800 grams is not gaining weight adequately (at 18 g/kg/day), fortification with human milk fortifier is recommended. Routine fortification of breast milk and post-discharge formula are not recommended after discharge of the neonate unless baby has suboptimal weight for the post-conception age. |
Level 1a |
References
2. Gidi NW, Goldenberg RL, Nigussie AK, McClure E, Mekasha A, Worku B, et al. Incidence and associated factors of extrauterine growth restriction (EUGR) in preterm infants, a cross-sectional study in selected NICUs in Ethiopia. BMJ Paediatrics Open. 2020;4(1).
3. Hay Jr WW. Optimizing nutrition of the preterm infant. Chinese Journal of Contemporary Pediatrics. 2017 Jan 25;19(1):1.
4. Makker K, Ji Y, Hong X, Wang X. Antenatal and neonatal factors contributing to extra uterine growth failure (EUGR) among preterm infants in Boston Birth Cohort (BBC). Journal of Perinatology. 2021 May;41(5):1025-32.
5. Cormack BE, Harding JE, Miller SP, Bloomfield FH. The influence of early nutrition on brain growth and neurodevelopment in extremely preterm babies: a narrative review. Nutrients. 2019 Sep;11(9):2029.
6. Moro GE, Arslanoglu S, Bertino E, Corvaglia L, Montirosso R, Picaud JC, et al. Human milk in feeding premature infants: consensus statement. Journal of Pediatric Gastroenterology and Nutrition. 2015 Sep 1;61(1):S16-9.
7. World Health Organization. Guidelines on optimal feeding of low birth-weight infants in low-and middle-income countries. World Health Organization; 2011.
8. Morgan J, Bombell S, McGuire W. Early trophic feeding versus enteral fasting for very preterm or very low birth weight infants. Cochrane Database of Systematic Reviews. 2013(3).
9. Morgan J, Young L, McGuire W. Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database of Systematic Reviews. 2014(12).
10. The SIFT Investigators Group. Early enteral feeding strategies for very preterm infants: current evidence from Cochrane reviews. Arch Dis Child Fetal Neonatal Ed. 2013; 98:F470–F472.
11. Sanghvi KP, Joshi P, Nabi F, Kabra N. Feasibility of exclusive enteral feeds from birth in VLBW infants>1200 g–an RCT. Acta Paediatrica. 2013 Jul;102(7):e299-304.
12. Bora R, Murthy NB. In resource limited areas complete enteral feed in stable very low birth weight infants (1000–1500 g) started within 24 h of life can improve nutritional outcome. The Journal of Maternal-Fetal & Neonatal Medicine. 2017 Nov 2;30(21):2572-7.
13. Nangia S, Bishnoi A, Goel A, Mandal P, Tiwari S, Saili A. Early total enteral feeding in stable very low birth weight infants: a before and after study. Journal of Tropical Pediatrics. 2018 Feb;64(1):24-30.
14. Walsh V, Brown JV, Copperthwaite BR, Oddie SJ, McGuire W. Early full enteral feeding for preterm or low birth weight infants. Cochrane Database of Systematic Reviews. 2020(12).
15. Salas AA, Kabani N, Travers CP, Phillips V, Ambalavanan N, Carlo WA. Short versus extended duration of trophic feeding to reduce time to achieve full enteral feeding in extremely preterm infants: an observational study. Neonatology. 2017;112(3):211-6.
16. Modi M, Ramji S, Jain A, Kumar P, Gupta N. Early aggressive enteral feeding in neonates weighing 750–1250 grams: a randomized controlled trial. Indian Pediatrics. 2019 Apr;56(4):294-8.
17. Leaf A, Dorling J, Kempley S, McCormick K, Mannix P, Linsell L, et al., Abnormal Doppler Enteral Prescription Trial Collaborative Group. Early or delayed enteral feeding for preterm growth-restricted infants: a randomized trial. Pediatrics. 2012;129:e1260–8.
18. Tewari VV, Dubey SK, Kumar R, Vardhan S, Sreedhar CM, Gupta G. Early versus late enteral feeding in preterm intrauterine growth restricted neonates with antenatal Doppler abnormalities: an open-label randomized trial. Journal of Tropical Pediatrics. 2018 Feb;64(1):4-14.
19. Arnon S, Sulam D, Konikoff F, Regev RH, Litmanovitz I, Naftali T. Very early feeding in stable small for gestational age preterm infants: a randomized clinical trial. Journal De Pediatria (Versão em Português). 2013 Jul 1;89(4):388-93.
20. Morgan J, Young L, McGuire W. Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database of Systematic Reviews. 2014(12).
21. Morgan J, Young L, McGuire W. Slow advancement of enteral feed volumes to prevent necrotizing enterocolitis in very low birth weight infants. Cochrane Database Syst Rev 2013;3:CD001241.
22. Oddie SJ, Young L, McGuire W. Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database of Systematic Reviews. 2021(8).
23. Chandramati J, Karakad P, Ponthenkandath S. Effects of early minimal enteral nutrition in preterm very low birth weight infants with absent or reversal of end diastolic flow in umbilical arteries (AREDF) before birth. Int J of Adv Res. 2019;(7):141-144.
24. Agostoni C, Buonocore G, Carnielli VP, De Curtis M, Darmaun D, Decsi T, et al. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. Journal of Pediatric Gastroenterology and Nutrition. 2010; 50(1): 85-91.
25. Abiramalatha T, Thomas N, Thanigainathan S. High versus standard volume enteral feeds to promote growth in preterm or low birth weight infants. Cochrane Database of Systematic Reviews. 2021(3).
26. Barrington KJ, Fortin‐Pellerin E, Pennaforte T. Fluid restriction for treatment of preterm infants with chronic lung disease. Cochrane Database of Systematic Reviews. 2017(2).
27. Yadav A, Siddiqui N, Debata PK. Two-hourly vs Three-hourly Feeding in Very Low Birth weight Neonates: A Randomized Controlled Trial. Indian Pediatr. 2021 Apr 15;58(4):320-324.
28. Razak A. Two-hourly versus three-hourly feeding in very low-birth-weight infants: A systematic review and meta-analysis. American Journal of Perinatology. 2020 Jul;37(09):898-906.
29. Ibrahim NR, Van Rostenberghe H, Ho JJ, Nasir A. Short versus long interval for bolus feeding in very preterm infants. Cochrane Database Systematic Reviewers 2021;(8).
30. Abiramalatha T, Thanigainathan S, Balakrishnan U. Re‐feeding versus discarding gastric residuals to improve growth in preterm infants. Cochrane Database of Systematic Reviews. 2019(7).
31. Kaur A, Kler N, Saluja S, Modi M, Soni A, Thakur A, et al. Abdominal circumference or gastric residual volume as measure of feed intolerance in VLBW infants. Journal of Pediatric Gastroenterology and Nutrition. 2015 Feb 1;60(2):259-63.
32. Abiramalatha T, Thanigainathan S, Ninan B. Routine monitoring of gastric residual for prevention of necrotising enterocolitis in preterm infants. Cochrane Database of Systematic Reviews. 2019(7).
33. Flint A, New K, Davies MW. Cup feeding versus other forms of supplemental enteral feeding for newborn infants unable to fully breastfeed. Cochrane Database of Systematic Reviews. 2016(8).
34. Watson J, McGuire W. Transpyloric versus gastric tube feeding for preterm infants. Cochrane Database of Systematic Reviews. 2013(2).
35. Foster JP, Psaila K, Patterson T. Non‐nutritive sucking for increasing physiologic stability and nutrition in preterm infants. Cochrane Database of Systematic Reviews. 2016(10).
36. Agakidou E, Karagiozoglou-Lampoudi T, Parlapani E, Fletouris DJ, Sarafidis K, Tzimouli V, et al. Modifications of Own Mothers’ Milk Fortification Protocol Affect Early Plasma IGF-I and Ghrelin Levels in Preterm Infants. A Randomized Clinical Trial. Nutrients. 2019 Dec;11(12):3056.
37. Arslanoglu S, Boquien CY, King C, Lamireau D, Tonetto P, Barnett D, et al. Fortification of human milk for preterm infants: update and recommendations of the European Milk Bank Association (EMBA) Working Group on Human Milk Fortification. Frontiers in Pediatrics. 2019 Mar 22;7:76.
38. Yang Y, Li Z, Yan G, Jie Q, Rui C. Effect of different doses of vitamin D supplementation on preterm infants–an updated meta-analysis. The Journal of Maternal-Fetal & Neonatal Medicine. 2018 Nov 17;31(22):3065-74.
39. McCarthy EK, Dempsey EM, Kiely ME. Iron supplementation in preterm and low-birth-weight infants: a systematic review of intervention studies. Nutrition Reviews. 2019 Dec 1;77(12):865-77.
40. Goldber DL, Becker PJ, Brigham K, Carlson S, Fleck L, Gollins L, et al. Definition of preterm nutrition; Sandrock, M.; Fullmer, M.; Van Poots, H.A. Identifying malnutrition in preterm and neonatal populations: Recommended indicators. J Acad Nutr Diet. 2018; 118:1571-1582.
41. Thanigainathan S, Abiramalatha T. Early fortification of human milk versus late fortification to promote growth in preterm infants. Cochrane Database of Systematic Reviews. 2020(7).
42. Brown JV, Embleton ND, Harding JE, McGuire W. Multi‐nutrient fortification of human milk for preterm infants. Cochrane Database of Systematic Reviews. 2016(5).
43. Alan S, Atasay B, Cakir U, Yildiz D, Kilic A, Kahvecioglu D, et al. An intention to achieve better postnatal in-hospital-growth for preterm infants: adjustable protein fortification of human milk. Early Human Development. 2013 Dec 1;89(12):1017-23.
44. Fabrizio V, Trzaski JM, Brownell EA, Esposito P, Lainwala S, Lussier MM, et al. Individualized versus standard diet fortification for growth and development in preterm infants receiving human milk. Cochrane Database of Systematic Reviews. 2020(11).
45. Young L, Embleton ND, McGuire W. Nutrient‐enriched formula versus standard formula for preterm infants following hospital discharge. Cochrane Database of Systematic Reviews. 2016(12).
46. Young L, Embleton ND, McCormick FM, McGuire W. Multi-nutrient fortification of human breast milk for preterm infants following hospital discharge. Cochrane Database of Syst Rev 2013;(2):CD004866.