Abstract
The craniovertebral junction is unique and the most complex of the axial skeleton in multiple aspects; embryology, anatomy, pathology and kinematics. A surgical physiological approach to management of its abnormalities was instituted at the University of Iowa Hospitals & Clinics (UIHC) in 1977 and has been the accepted treatment algorithm worldwide. A global referral led to a prospective database of more than 7,000 adult and pediatric patients; 3,600 of whom were treated at UIHC. 975 children (less than 18 years of age) were surgically managed. The commonest symptoms and signs were neck pain and headache, neurological deficit due to brain stem and spinal cord compression, vascular issues secondary to vertebral artery compression and the Chiari malformation.
Keywords
Craniovertebral junction, Atlantoaxial dislocation, Basilar invagination, Fiberoptic intubation, Intraoperative reduction of cervicomedullary compression, Klippel-Feil syndrome, Anesthesia
Introduction
Until the 1970s, the treatment of abnormalities of the craniovertebral junction (CVJ) consisted of posterior fossa decompression which had a morbidity and mortality of 30% [1]. In 1977, the Department of Neurosurgery at the University of Iowa Hospitals & Clinics (AHM) adopted a more (patho-)physiological surgical approach based on an understanding of the craniocervical dynamics, the site of encroachment and the stability of the craniovertebral junction [2]. Table 1 provides an easy reference to the classification of the craniovertebral junction abnormalities that we have encountered. Craniovertebral abnormalities can be classified as reducible and irreducible lesions [1-3]. Reducibility refers to the ability to restore anatomical alignment (by flexion/extension/traction) thereby relieving compression of the cervicomedullary junction (CMJ). The treatment algorithm explains the decision tree (Table 2). In irreducible lesions, an attempt is made to achieve reduction intraoperatively under general anesthesia with neuromuscular blockade using crown halo traction; O-arm CT is used to confirm reduction [4]. When this maneuver results only in a partial reduction, complete reduction can be achieved by manipulation of the atlantoaxial joint. In reducible lesions, alternatively, a dorsal operative stabilization is necessary. This manuscript refers to the management of 975 children with CVJ pathology.
|
I. Congenital anomalies and malformations A. Occipital sclerotome malformations e.g. proatlas remnants, clivus segmentations, condylar hypoplasia, atlas assimilation B. Atlas malformations e.g. bifid atlas, assimilation, fusions, absent arches C. Axis malformations e.g., segmentation defects, odontoid dysplasias II. Developmental and acquired abnormalities A. Foramen magnum abnormalities 1. Foramen stenosis e.g., achondroplasia 2. Secondary invagination e.g., osteogenesis imperfecta, Hadju-Cheney, renal rickets, Paget’s B. Atlantoaxial instability 1. Down’s syndrome 2. Errors of metabolism e.g., Morquio’s, Hurlers 3. Infections e.g. Grisel’s syndrome, tuberculosis 4. Trauma 5. Inflammation e.g. regional ileitis, juvenile rheumatoid arthritis, Reiter’s syndrome 6. Tumors e.g. osteoblastoma, eosinophilic granuloma, chordoma, neurofibromatosis 7. Miscellaneous e.g. Conradi syndrome, fetal warfarin, syringomyelia |
Table 2: Treatment algorithm.
Symptoms and Signs
The most common symptom in 68% of the children, was pain in the posterior cervical region secondary to compression of the C2 nerve root [1,5]. Compression of the brain stem, lower cranial nerves and/or the spinal cord led to neurological dysfunction in 45% of patients. Distortion or compression of the vertebral artery was confirmed in 26%, while 28% of children had Chiari I malformation with its attendant symptoms and signs.
All patients underwent neurodiagnostic imaging with cervical spine radiographs, CT and MRI of the brain and spine using T2 mode in flexion and extension to identify the lesion as well as determine its reducibility [1]. Children with brain stem and lower cranial nerve symptoms were evaluated with preoperative swallow studies and pulmonary function tests. There were 975 children ranging in age from 6 months to 18 years with an average age of 11 years. Illustrative cases, along with the anesthetic considerations for each one, are shown in Table 3.
|
Age/Sex |
Radiographic Pathology |
Symptoms/Signs |
Surgical Management |
Anesthesia Consideration |
|
Case #1 – Figure 1
11/F |
|
|
|
|
|
Case #2 – Figure 2
7/F |
|
|
|
|
|
Case #3 – Figure 3
14/M |
|
|
|
|
|
Case #4 – Figure 4
10/M |
|
|
|
|
|
Case #5 – Figure 5
11/F |
|
|
|
|
|
Case #6 – Figure 6
18/M |
|
|
|
|
|
CN: Cranial Nerves; SSEP: Somatosensory Evoked Potentials; MEP: Motor Evoked Potentials; ET: Endotracheal Tube; NG: Nasogastric; GA: General Anesthesia |
||||
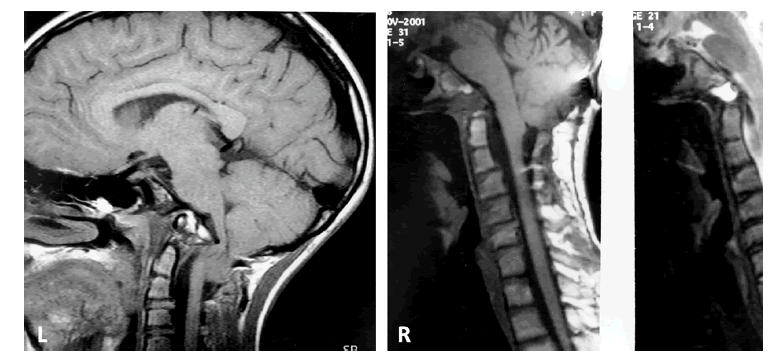
Figure 1: Case #1; Composite of preoperative (L) T1-W MRI of craniovertebral junction and postoperative (R) studies. The ventral indentation of the medulla is severe with cerebellar tonsils to C2 (L). The postoperative views (R) show decompression of medulla and cerebellar tonsillar ascent (T1-W and T2-W MRI).
Figure 2: Case #2; Composite of preoperative (L) and postoperative (R) T2-W MRI of craniocervical junction. There is a proatlas invagination into the ventral medulla (L), hindbrain herniation and cervical syringohydromyelia. The postoperative (R) image shows relief of ventral medullary compression, syrinx decompression and ascension of cerebellar tonsils.
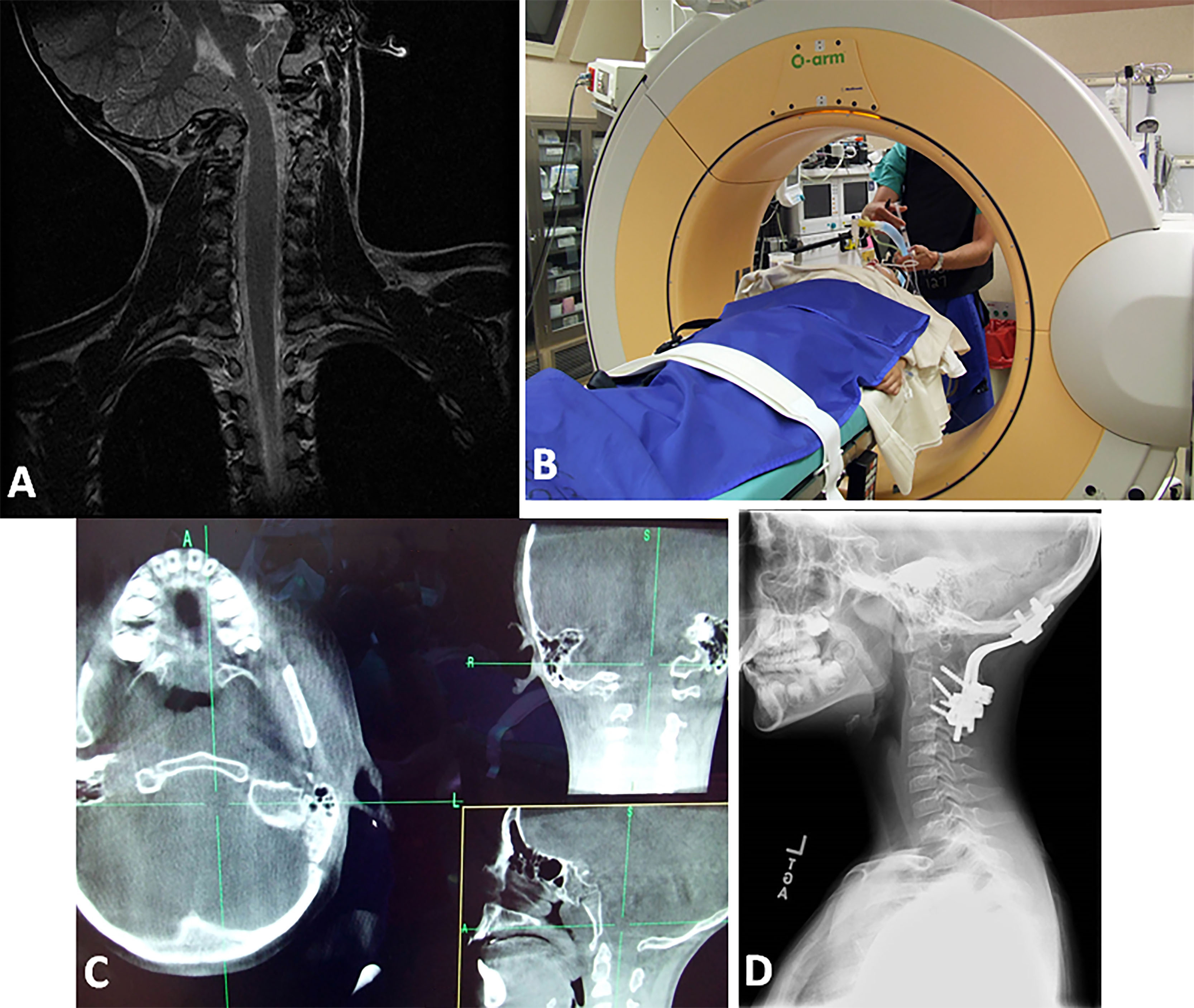
Figure 3: Case #3; A) Awake fiberoptic intubation with “Miami J” collar in place (X). B) Placement of crown halo for intraoperative traction. The head is stabilized during the procedure (chin fixed) and the posterior collar shell is in place. C) Operative photograph of dorsal C1-C2 arthrodesis with screws and rib grafts. D) Postoperative lateral cervical spine radiograph reveals C1 lateral mass screws and C2 pars screws with interscrew rod fixation.
Figure 4: Case #4; A) Coronal T2-W MRI of craniocervical region. Note the O-C1 dislocation with cervicomedullary distortion. B) Anesthetized patient in O-arm CT in traction. C) Composite of axial, coronal and sagittal plane CT in the “O-arm” CT in traction. The rotary dislocation is reduced. D) Postoperative lateral cervical radiograph. There is a completed dorsal occipitocervical fusion.
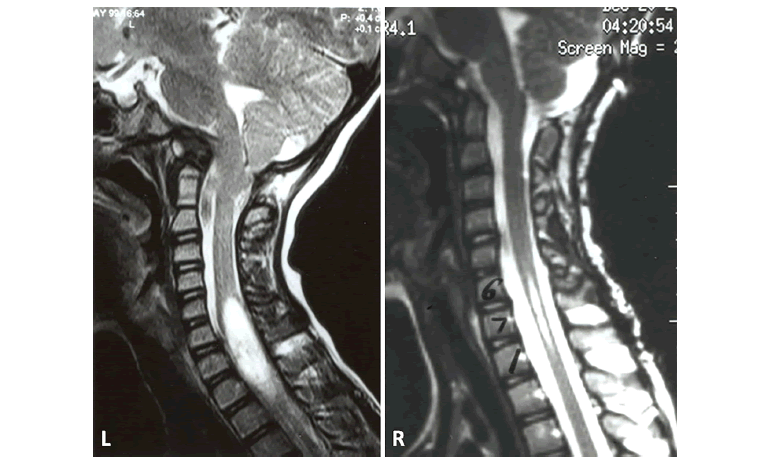
Figure 5: Case #5; A) 3D CT of CVJ in lateral projection. Note the atlantoaxial dislocation. B) 3D CT of CVJ viewed from below. There is a rotary atlantoaxial luxation (jumped facet) (arrow). C) Axial T1-W MRI at C1-C2 level. Arrow points the disrupted transverse cruciate ligament. D) Intraoperative lateral craniocervical fluoroscopic image. The patient is in crown halo traction. The ET tube angle is > 110 degrees (arrows). The dorsal O-C2 fusion is made.
Figure 6: Case #6; Lateral CVJ T1-W MRI in midsagittal plane. There is atlas assimilation, severe odontoid invagination into the upper medulla, cerebellar tonsils to C2-3, and evidence of posterior fossa decompression with C1-C3 laminectomies.
Discussion
The Neurosurgery, Anesthesia and Pediatric Intensive Care Teams at the University of Iowa form one cohesive multi-disciplinary group [6,7]. Each case, including the presence and extent of instability as well as brainstem compression and potential perioperative pitfalls, is reviewed before surgery. The importance of maintaining the optimal position of the head and neck at all times, preoperatively, intraoperatively and postoperatively, is reiterated to the team.
Bracing, preoperative and intraoperative surgical and anesthetic considerations:
- Craniovertebral abnormalities in children below the age of 4 years are managed using a brace with the exception of children with severe neurological dysfunction who require an immediate operation. Ventral surgical procedures are performed with the patient in traction in the supine position while the dorsal operation is performed with the child in traction in the prone position. Postoperative immobilization is provided by an occipito-cervical Minerva molded brace.
- The nutritional status of the patient is assessed preoperatively. Poor nutrition has implications for postoperative management and recovery [1,6].
- Neuroendocrine problems (e.g., hypothyroidism) in patients with Down syndrome need to be assessed and managed preoperatively.
- Preoperative education of both the child (tailoring the information provided to the level of understanding) and the parents/guardians is critical for reducing the anxiety and apprehension of both. This is particularly essential if an awake intubation is planned (see below).
- During the clinic visit, it is important to elicit a history of gastroesophageal reflux; if present, preoperative swallow studies [1] must be obtained. Intact pharyngeal and tongue sensation in addition to the presence of a gag reflex must be ascertained [1]. An intact gag reflex demonstrates the integrity of the hypoglossal and vagus nerves. The risk of aspiration of gastric contents should be considered; we prescribe a proton pump inhibitor preoperatively as well as postoperatively [6]. Precautions to prevent aspiration are also taken during the induction of anesthesia, for instance, keeping tidal volumes and pressures low during mask ventilation to minimize gastric insufflation, and decompressing the stomach using an orogastric tube once the patient’s airway is secured.
- The cardiovascular and pulmonary status must be evaluated preoperatively and optimized, with referral to sub-specialists as needed.
- After the induction of anesthesia, prophylactic antibiotics and dexamethasone to reduce airway edema are administered.
- When there is preoperative brain stem compression, age-appropriate mean arterial blood pressure must be maintained intraoperatively-an average target MAP being 70-75 mmHg for ages 8-12. Depending on the age and maturity of the patient, asleep or awake fiberoptic-guided intubation, rather than direct or video laryngoscopy, is preferable. Children older than 10 years of age with an unstable cranio-cervical spine can usually cooperate with awake fiberoptic-aided intubation. Thoroughly anesthetizing the airway is critical for successful awake intubation. A detailed description of the technique of fiberoptic intubation is outside the scope of this article. However, several points need emphasis [6-9]. First an acceptable neck position has to be decided upon by the surgical team and the neck must be braced in that position. The cervical collar must be in place during the intubation. During the transoral approach, a cuffed endotracheal tube is necessary to prevent aspiration of blood. A wire-reinforced endotracheal tube stays deformed after compressive force is released instead of springing back to patency. Hence, wire-reinforced endotracheal tubes should be avoided in patients who will undergo transoral decompression because of possible compression of the tube by the lingual blade of the oral retractor system.
- In a child with craniocervical instability, a cervical collar is maintained during the induction of general anesthesia and intubation. In younger children, a laryngeal mask airway (LMA) can be used to ventilate and a fiberoptic intubation performed through the LMA. The LMA allows for better ventilation than a face mask in most cases and serves as a conduit for the fiberoptic scope and endotracheal tube. The endotracheal tube can be inserted through the LMA and connected to the Anesthesia circuit. Addition of a bronchoscope adapter to the endotracheal tube end allows introduction of the fiberoptic bronchoscope while the patient continues to be ventilated. More recently, videolaryngoscopy has become widely used for intubation. While performing laryngoscopy, maximal movement of the neck occurs during extension and the degree of extension is significantly greater during laryngoscopy compared to that during a fiberoptic-guided intubation. Hence clinical judgment should be used to determine when videolaryngoscopy can be used safely over a fiberoptic intubation. After intubation, optimal depth of the endotracheal tube should be confirmed using a fiberoptic bronchoscope. Thereafter, crown halo traction is placed.
- The following 2 paragraphs address complications that can occur during the prone position and their avoidance. When the child is positioned prone for the fusion, cervical traction is maintained initially in a neutral position with the crown halo resting on a Mayfield horseshoe headrest and the angle of the endotracheal tube (between the oral cavity and larynx) is carefully checked. If the angle is less than 90-100 degrees at the time of the craniocervical dorsal fusion, the neck will be fused at a more acute angle and the patient is likely to have difficulty breathing and swallowing postoperatively [5,10]. Intraoperatively, with extreme neck flexion, the endotracheal tube can become kinked, result in higher peak airway pressures and difficulty in ventilating the patient. Hence the “Army tuck” or chin tuck position is avoided and intraoperative cervical alignment verified by fluoroscopy. This should be done prior to the start of the surgical skin prep.
Other anesthetic complications associated with the prone position [11] include dislodgement or malposition of the endotracheal tube or vascular catheters, difficult ventilation due to decreased excursion of the chest or abdominal compression (the latter can also cause hypotension), pressure injuries or neuropathies, facial and airway edema, and rarely, blindness due to retinal artery thrombosis or ischemic optic neuropathy. Auscultation of the lungs immediately after rendering the patient prone will ascertain maintenance of correct endotracheal tube placement. Pressure points must be checked and padded. Additionally, the patency of intravenous and arterial catheters must be verified before surgical drapes limit the anesthesiologist’s access. - For irreducible lesions at the craniocervical junction, general anesthesia is induced followed by crown halo traction. Intraoperative neurophysiological monitoring, namely somatosensory and motor evoked potentials (SSEPs and MEPs), is conducted. The child is then positioned in the O-arm CT and neuromuscular blockade is initiated at this point, prior to imaging. To confirm further reducibility, the O-arm CT series is performed in the supine as well as the prone positions with cervical traction. If the lesion is still irreducible, a ventral surgical decompression is performed. Intravenous anesthetics with or without a low concentration of a volatile agent are commonly used during somatosensory and motor evoked potential monitoring. Muscle relaxants are helpful during reduction of the lesion but are discontinued afterwards when motor evoked potentials are being monitored. The use of an EEG-based depth-of-anesthesia monitor (such as BIS or Entropy monitors) may be considered.
- If the lesion is reducible, a dorsal fusion is made. All the above precautions remain.
- During a lateral extrapharyngeal approach, when the patient’s head is extended, the upper and lower teeth can abut, causing occlusion of oral endotracheal tubes. To prevent airway obstruction, nasal intubation is mandated when using this particular surgical approach.
- After a transoral CVJ decompression, the patient should remain intubated for 3-4 days postoperatively. Acute airway obstruction can occur due to prevertebral swelling and/or changes in the occipitocervical angle. Additionally, intraoperative fluids administered in the prone position may contribute to facial and laryngopharyngeal swelling and further delay extubation. It is critical for the critical care, anesthesiology and otolaryngology teams to be aware of the presence of these patients on the wards. Team members must be cognizant of the potential for life-threatening loss of the airway if they are prematurely extubated (either self-extubation or by clinicians) and require reintubation. Mask ventilation and/or endotracheal intubation can be exceedingly difficult due to changes after surgical CVJ fusion, tissue swelling and retained secretions. Once a patient meets routine extubation criteria, the anesthesiologist and the neurosurgeon must review the extent of prevertebral swelling on lateral cervical radiographs and verify the presence of a leak around the deflated endotracheal tube cuff. Only then should extubation be considered. Removal of the endotracheal tube over a tube exchange catheter is recommended so that reintubation can be easily accomplished. These patients should only be extubated during a period of the day when expert airway assistance is readily available; the anesthesiologist and otolaryngologist should be at the bedside during extubation. We (RNM and AHM) created a difficult extubation protocol including bold signage by the bedside, a bright wristband and prominent red tape on the endotracheal tube.) Among our patient cohort, delayed extubation was performed in the operating room in 6 patients who were considered to be especially difficult airways post-procedure. Three of these six patients required tracheostomy in the operating room after failed extubation and inability to reintubate (Table 4).
We consider it critically important that each craniovertebral fusion patient wears a medical alert bracelet (or other tag) after surgery that states “Craniovertebral Junction Fusion – Difficult Intubation.” This alert could be life-saving should these patients require emergent intubation or surgery at another institution.
|
A. Patient Population • Difficult intubation in operating room for craniovertebral junction cases. • Patients with prior transoral procedures or occipitocervical or extensive posterior cervical spine fusion (surgical or spondylotic) • Any other intubated patient whom the anesthesiologist, otolaryngologist or surgeon feels may be a difficult or dangerous extubation/reintubation |
|
B. Pre-extubation steps/conditions days before planned extubation • Chest x-ray – no infiltrates • Lateral cervical spine radiographs to evaluate prevertebral swelling • Stop nasogastric tube feedings 8 hours prior • Cultures of endotracheal secretions • Check for cuff leak with endotracheal tube cuff deflated. • IV Decadron 2 hours before extubation • If no contraindications exist, glycopyrrolate, an anti-sialogogue, may be administered to the patient 30-45 minutes before the extubation. • All sedation discontinued, soft cervical collar in place • Meets all other routinely used criteria for readiness to extubate • During the daytime with the anesthesiologist, intensivist, neurosurgeon and otolaryngologist present along with a fiberoptic bronchoscope and other airway aids for ventilating and reintubating ready |
|
C. Extubation • The endotracheal tube is removed over an airway exchange catheter in-situ for approximately 45 minutes or until clinicians consider it safe to do so. If there are no problems and the patient can breathe adequately at the end of that period without the endotracheal tube, the airway exchange catheter is removed. Otherwise, the endotracheal tube is replaced over the airway exchange catheter. • If there is a high probability that extubation may fail and it is deemed necessary, it should occur in the operating room with the site prepped and appropriate staff and equipment present for performing a surgical airway. |
|
D. Post-extubation • Follow the adequacy of respiratory (oxygenation and ventilation) and swallowing in intensive care unit for 48 hours with the readiness to reintubate safely if necessary. • May need racemic epinephrine nebulization if there are signs of upper airway edema during the 24-hour period immediately following extubation. • Wrist band (in the hospital and also thereafter), medical chart alert, and signs by the bedside to note “craniocervical fusion – difficult intubation – needs awake fiberoptic intubation” |
Conclusion
The anesthesia team plays an integral part in the care of patients with abnormalities of the craniovertebral junction. Communication between the neurosurgeon, anesthesiologist and intensivist throughout the perioperative period is imperative for an optimal outcome.
Conflicts of Interest
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Acknowledgments
We acknowledge Mary Jo Piper for her assistance in formatting the paper.
References
2. Menezes AH. Craniovertebral junction database analysis. Incidence, classification, presentation and treatment algorithms. Childs Nerv Syst 2008;24:1101-1108.
3. Menezes AH, Sonntag VKH. Principles of Spinal Surgery. New York: McGraw-Hill; 1996. p. 1-1525.
4. Dahdaleh NS, Dlouhy BJ, Menezes AH. Application of neuromuscular blockade and intraoperative 3D imaging in the reduction of basilar invagination. J Neurosurg Pediatr 2012;9:119-124.
5. Menezes AH, Dlouhy BJ. Atlas assimilation: Spectrum of associated radiographic abnormalities, clinical presentation and management in children below 10 years. Childs Nerv Syst 2020;36:975-985.
6. Maktabi MA. Anesthetic management of CVJ surgical procedures, particularly transoral procedures. Oper Tech Neurosurg 2005;8:143-149.
7. Menezes AH, Szeluga DJ. Postoperative management of transoral approaches to the craniovertebral junction. Oper Tech Neurosurg 2005;8:158-159.
8. Sawin PD, Todd MM, Traynelis VC, Farrell SB, Nader A, Sato Y, et al. Cervical spine motion with direct laryngoscopy and orotracheal intubation. An in vivo cinefluoroscopic study of subjects without cervical abnormality. Anesthesiology 1996;85(1):26-36.
9. Johnson DM, From AM, Smith RB, From RP, Maktabi MA. Endoscopic study of mechanisms of failure of endotracheal tube advancement into the trachea during awake fiberoptic orotracheal intubation. Anesthesiology 2005;102(5):910-914.
10. Smith-Hammond CA, New KC, Pietrobon R, Curtis DJ, Scharver CH, Turner DA. Prospective analysis of incidence and risk factors of dysphagia in spine surgery patients: Comparison of anterior cervical, posterior cervical and lumbar procedures. Spine (Phila PA 1976) 2004;29:1441-1446.
11. Mueller RN. Anesthesia for Posterior Fossa Surgery. Fundamentals of Neuroanesthesia: A Physiologic Approach to Clinical Practice. Oxford University Press. 2013. p. 209-222.
12. Mueller RN, Todd MM, Kacmarynski D, Menezes AH. Extubation protocol for pediatric patients after posterior craniocervical fusion. Department of Anesthesia, University of Iowa Hospitals and Clinics, 2014, unpublished.