Abstract
Expanding upon the applications of artificial intelligence (AI) explored in “Artificial Intelligence for Drug Repurposing Against Infectious Diseases,” this commentary explores AI’s transformative potential in hematology. AI-driven algorithms are revolutionizing diagnostics through the automation of tasks like blood smear analysis, cell classification, flow cytometry, and early disease detection. By leveraging extensive datasets, these algorithms enhance accuracy and efficiency in identifying patterns, classifying cells, detecting abnormalities, and predicting disease progression. In the realm of therapeutics, AI is reshaping personalized medicine by analyzing patient data to tailor treatment strategies. AI-powered platforms are accelerating drug discovery, optimizing clinical trial design, and enabling real-time treatment monitoring and personalized risk assessment. While challenges such as algorithm transparency, data bias, and ethical considerations remain, the future of AI in hematology is promising. Continued research, collaboration, and responsible implementation are essential to fully harness AI’s potential for improving patient care and advancing therapeutic interventions.
Keywords
Artificial intelligence, Hematology, Machine learning, Deep learning, Diagnostics, Therapeutics, Algorithm transparency, Data bias, Ethics
Introduction
The increasing prevalence of drug-resistant pathogens has highlighted the need for innovative approaches in drug discovery, including drug re-purposing. Artificial intelligence (AI) has emerged as a transformative tool in this endeavor, with the potential to revolutionize not only drug re-purposing for infectious diseases but also the field of hematology [1].
Hematology, the study of blood and blood disorders, has long relied on meticulous manual analysis and interpretation of microscopic images, flow cytometry data, and genetic information. However, the advent of artificial intelligence (AI) has opened up new avenues for automating and enhancing various aspects of hematological practice, from diagnosis and prognosis to treatment selection and monitoring. AI, a multidisciplinary field that simulates human cognitive processes, encompasses a range of computational techniques, with machine learning (ML) and deep learning (DL) being particularly relevant to hematology. ML algorithms, through data analysis, identify patterns and generate predictions, while DL, a subset of ML, utilizes artificial neural networks to model intricate data relationships [1]. In hematology, AI is being employed for diverse applications, including image analysis, identifying and classifying blood cells, detecting subtle morphological abnormalities, data interpretation, risk stratification, and treatment optimization [2-4]. However, the integration of AI into hematological practice also raises critical questions and challenges. One of the main concerns is the potential for bias and errors in AI algorithms, which could lead to misdiagnosis or inappropriate treatment decisions. The interpretability and explainability of AI models also remain a challenge, as it is often difficult to understand the underlying reasoning behind their predictions. Moreover, the ethical and legal implications of using AI in healthcare, such as issues of accountability, transparency, and patient consent, need to be carefully considered. Despite these challenges, the potential benefits of AI in hematology are immense. By automating tedious and time-consuming tasks, AI can free up healthcare professionals to focus on more complex and patient-centered activities. AI algorithms can also potentially improve the accuracy and efficiency of diagnosis and prognosis, leading to better patient outcomes. However, it is crucial to adopt a critical perspective and carefully evaluate the risks and benefits of AI in hematology to ensure its responsible and ethical implementation. This paper aims to provide a critical overview of the current state of AI in hematology, highlighting its potential benefits and challenges. By examining the existing literature and exploring the ethical and social implications of AI in hematological practice, this paper aims to contribute to a more informed and nuanced understanding of this rapidly evolving field.
AI in Hematological Diagnostics
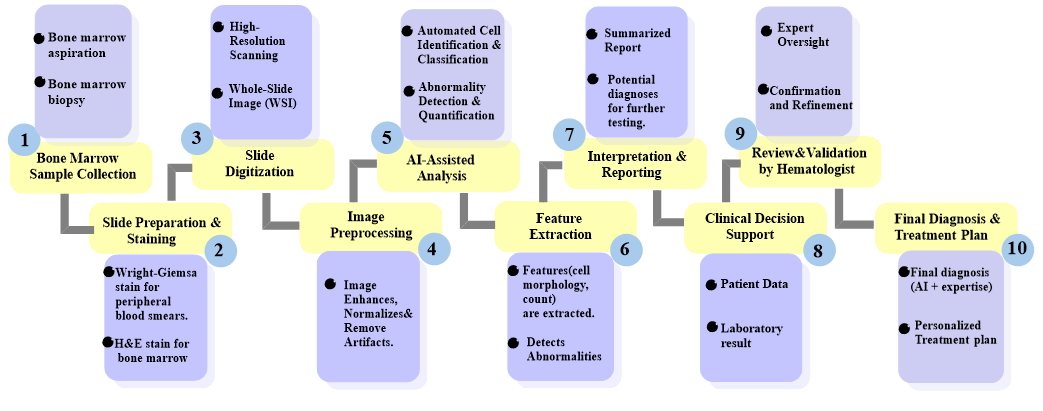
The integration of AI into hematological diagnostics has transformed the traditional workflow, as depicted in Figure 1. AI-powered tools are now seamlessly incorporated into various stages of the diagnostic process, from sample preparation and analysis to result interpretation and reporting. This streamlined workflow leverages AI algorithms (as summarized in Table 1), alongside traditional methods, to enhance the accuracy, efficiency, and overall effectiveness of hematological diagnosis. AI algorithms are employed for a wide range of tasks, including blood smear analysis, cell counting and classification, flow cytometry analysis, and early detection of hematological malignancies. By leveraging vast datasets, these algorithms identify intricate patterns, classify cells with precision, detect subtle abnormalities, and predict disease progression, ultimately leading to more informed and timely clinical decisions [5].
Figure 1: AI-powered Hematological Diagnosis Workflow.
|
AI Algorithm |
Applications in Hematological Diagnostics |
|
Convolutional Neural Networks (CNNs) |
Cell classification, abnormality detection, disease prediction (e.g., leukemia) [6] |
|
Recurrent Neural Networks (RNNs) |
Time-series analysis of blood cell counts and prediction of disease progression [7] |
|
Support Vector Machines (SVMs) |
Classification of hematological malignancies, differentiation of cell types [8]. |
|
Random Forests |
Feature selection for disease prediction, classification of blood disorders [9] |
|
Gradient Boosting Machines (GBMs) |
Prediction of treatment response, risk stratification of patients [10] |
|
Autoencoders |
Anomaly detection in blood cell morphology, dimensionality reduction for data visualization [11] |
|
Generative Adversarial Networks (GANs) |
Generation of synthetic blood smear images for data augmentation, anomaly detection [12] |
|
Deep Reinforcement Learning |
Optimization of treatment protocols for hematological disorders [13] |
|
Natural Language Processing (NLP) |
Extraction of information from clinical notes and reports, prediction of disease outcomes [14] |
|
Graph Neural Networks (GNNs) |
Analysis of protein-protein interaction networks in hematological malignancies, drug target identification [15] |
AI assisted hematologic cytology
AI is revolutionizing hematologic cytology by addressing the limitations of traditional manual microscopy, which is time-consuming, subjective, and prone to inter-observer variability [5]. AI algorithms, trained on extensive datasets of digitized blood and bone marrow smears, offer rapid and accurate analysis, efficiently identifying and classifying diverse cell types, including immature and abnormal cells, thus aiding in the diagnosis of hematological malignancies and other blood disorders [16]. AI-powered systems such as CellaVision and Morphogo have shown high accuracy in classifying various blood cells, detecting abnormalities, and predicting disease prognosis [16]. Chen et al. further exemplify AI's potential with Morphogo, an AI-powered system that accurately identifies and classifies circulating plasma cells (CPCs), crucial biomarkers for multiple myeloma (MM) diagnosis and monitoring [17]. This innovative approach surpasses the limitations of manual microscopy, enabling early and precise CPC detection through morphological examination, thereby facilitating accessible and efficient CPC screening for MM patients [17]. AI-based platforms like Scopio and Mantiscope enhance efficiency and throughput by automating slide digitization and cell identification [16]. Additionally, AI is proving valuable in diagnosing challenging cases, such as differentiating myelodysplastic syndromes from aplastic anemia and identifying acute myeloid leukemia subtypes [16]. The integration of AI with immunohistochemical staining further enhances the accuracy and efficiency of lymphoma diagnosis [16]. These advancements underscore AI's potential to transform hematologic cytology by providing faster, more accurate, and standardized diagnostic tools.
AI assisted image analysis
The interpretation of blood smears, a cornerstone of hematological diagnostics, is undergoing a transformation due to the advent of automated image analysis powered by machine learning (ML) algorithms [18]. Convolutional neural networks (CNNs), a type of deep learning model, have emerged as a powerful tool in this domain, demonstrating remarkable accuracy in identifying and classifying various blood cell types. Recent research has shown that CNNs can achieve accuracy levels comparable to, or even surpassing, those of experienced hematologists. For instance, a study reported a CNN model that achieved 98.5% accuracy in classifying white blood cells, outperforming human experts who achieved 95% accuracy [19]. This advancement in image analysis is not only accelerating the diagnostic process but also reducing inter-observer variability, a common challenge in manual microscopy. By providing consistent and objective assessments, AI-powered image analysis is enhancing the reliability and re-producibility of blood smear interpretations [20]. Moreover, the ability of AI algorithms to rapidly analyze large numbers of cells is facilitating the early detection of hematological malignancies such as leukemia, myelodysplastic syndromes (MDS), and anemias [21]. A CNN-based system was able to detect MDS with a sensitivity of 92% and a specificity of 96%, significantly improving early diagnosis rates [22]. Beyond the fundamental tasks of identifying and classifying blood cells, AI algorithms are expanding their capabilities to quantify intricate cellular features like size, shape, and granularity. This quantitative analysis is proving to be a game-changer in hematology, offering deeper insights into the underlying mechanisms of diseases and their progression. By precisely measuring and analyzing these cellular characteristics, researchers and clinicians can gain a more comprehensive understanding of how diseases develop and evolve at the cellular level. This newfound ability to quantify cellular features is opening doors to the discovery of novel biomarkers and therapeutic targets. Biomarkers are measurable indicators of biological processes or disease states, and their identification can lead to earlier and more accurate diagnoses, as well as the development of targeted therapies. For instance, in sickle cell disease, a hereditary blood disorder characterized by abnormally shaped red blood cells, AI-powered analysis of red blood cell morphology has shown promise in predicting the risk of complications such as vaso-occlusive crises and acute chest syndrome. By analyzing subtle variations in red blood cell shape, size, and texture, AI algorithms can identify patients at higher risk of these complications, allowing for proactive interventions and personalized treatment plans [23]. In addition to sickle cell disease, AI-powered quantification of cellular features is being explored in various other hematological conditions. For example, in myelodysplastic syndromes (MDS), a group of disorders characterized by abnormal blood cell production, AI algorithms are being used to analyze the morphology of bone marrow cells to predict disease progression and response to treatment. In acute myeloid leukemia (AML), AI-powered analysis of blast cell morphology is aiding in the identification of distinct sub-types with different prognoses and treatment responses [24-25]. Recent advancements in AI algorithms have expanded their application beyond blood cells to include platelet morphology analysis [26]. These algorithms, particularly CNNs, have shown promise in predicting bleeding and thrombosis risk [27]. Deep learning-enabled technologies have further improved the quantification and classification of cellular features, including platelet morphology [28]. The University of Birmingham has developed an adaptable analysis workflow for platelet spreading and morphology, which includes image segmentation and machine learning methods for automated classification [29]. These developments collectively highlight the potential of AI in enhancing platelet analysis and its clinical implications.
AI assisted flow cytometry
Flow cytometry, a cornerstone of hematological diagnostics, generates multi-parametric data crucial for identifying and quantifying cell populations. However, the manual interpretation of this data can be time-consuming and prone to subjectivity. Artificial intelligence (AI) is transforming flow cytometry analysis by automating and enhancing various aspects of the process. AI-powered algorithms, trained on vast datasets of immunophenotypic profiles, are enabling rapid and accurate identification of cell populations. This is particularly valuable in the diagnosis and classification of hematological malignancies such as leukemias, lymphomas, and myelodysplastic syndromes [30]. Recent studies have demonstrated the potential of deep learning models in accurately classifying different types of acute leukemia based on flow cytometry data. For instance, Cheng and Lewis both reported high sensitivity and accuracy in detecting and classifying acute myeloid leukemia (AML) and B-lymphoblastic leukemia (B-ALL) using deep learning models [31,32]. Monaghan (2021) further expanded on this by developing a machine learning model that could rapidly distinguish between different types of acute leukemias and nonneoplastic cytopenias, achieving high accuracy [33]. Furthermore, AI is not only automating routine analysis but also uncovering novel insights. Unsupervised clustering algorithms, a type of AI technique, can identify previously unknown cell subsets or disease patterns within flow cytometry data. This has the potential to reveal new diagnostic biomarkers or therapeutic targets, paving the way for more precise and personalized treatment approaches [34].
AI assisted genomic analysis
Next-generation sequencing (NGS) technologies have transformed genomic analysis in hematology, generating large volumes of data that necessitate advanced computational tools for interpretation. AI algorithms, particularly deep learning models, are adept at identifying pathogenic mutations, predicting disease risk and prognosis, and guiding therapeutic decision-making. In hematological malignancies, AI-powered analysis of genomic data aids in risk stratification, identifying patients who may benefit from targeted therapies or allogeneic stem cell transplantation [35,36]. Advanced models, such as genome-scale metabolic modeling and deep generative models for graphs, are enhancing the analysis of biological networks within pathogens and their human hosts, leading to the identification of crucial drug targets [37]. A personalized prediction model integrating clinical and genomic data was developed, demonstrating high accuracy in predicting survival and leukemia transformation probabilities for individual myelodysplastic syndromes (MDS) patients. This model identified key prognostic factors, including chromosomal karyotype, blood cell counts, bone marrow blast percentage, age, and specific gene mutations. Validation across multiple independent cohorts confirmed its superior performance compared to established prognostic models [38].
AI assisted clinical decision support in Hematology
AI-powered clinical decision support (CDS) systems integrate patient data from diverse sources, including laboratory results, imaging studies, and electronic health records (EHRs), to provide clinicians with personalized diagnostic and therapeutic recommendations. In hematology, CDS systems can suggest differential diagnoses, predict disease progression, and recommend optimal treatment regimens based on patient-specific characteristics and disease profiles. These systems have the potential to improve diagnostic accuracy, optimize therapeutic decision-making, and ultimately enhance patient outcomes [39]. This is particularly evident in the field of hematological malignancies, where AI platforms like Watson for Genomics have demonstrated high concordance with manual interpretation and identified clinically actionable insights [40]. However, the use of AI in this context also presents challenges such as the need for validation and standardization, potential data privacy issues, and the risk of systematic errors and bias.
AI in Hematological Therapeutics
The integration of AI in hematological therapeutics is rapidly transforming the landscape of disease management and drug development. Leveraging complex algorithms and computational power, AI is revolutionizing personalized medicine, drug discovery, clinical trial design, and treatment monitoring, with the goal of improving patient outcomes and accelerating therapeutic innovation.
Personalized medicine
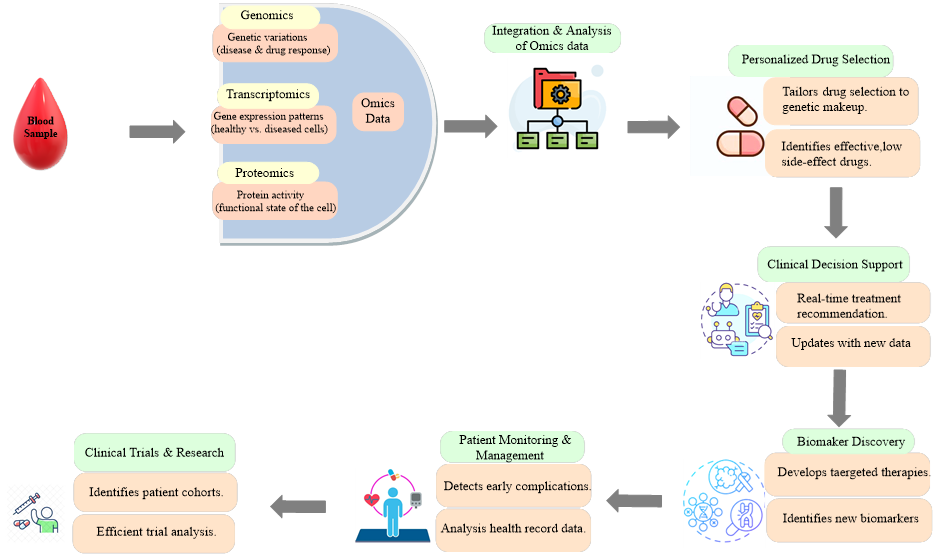
AI-driven personalized medicine in hematology aims to tailor treatment strategies to individual patient characteristics, thereby optimizing efficacy and minimizing adverse events. The integration of AI with technologies like CRISPR-Cas9 gene editing may enable the development of personalized therapies tailored to individual patients and specific pathogen strains. Machine learning algorithms analyze vast datasets, integrating patient demographics, clinical parameters, genetic profiles, and treatment responses to identify patterns and predict individual responses to therapy. This enables clinicians to select the most appropriate treatment modality for each patient, considering factors such as disease sub-type, molecular profile, comorbidities, and prior treatment history [41]. For instance, in acute myeloid leukemia (AML), AI algorithms have been developed to predict patient responses to chemotherapy and allogeneic stem cell transplantation, facilitating risk stratification and individualized treatment decisions [42]. Figure 2 provides a schematic representation of how AI integrates multi-omics data (genomic, transcriptomic, proteomic) with clinical information to predict treatment response and guide personalized treatment decisions for hematological disorders.
Figure 2: AI-driven personalized treatment in hematology.
Drug discovery and development
AI is playing an increasingly pivotal role in accelerating drug discovery and development in hematology. Traditional drug discovery pipelines are often labor-intensive, time-consuming, and expensive. AI algorithms, however, can rapidly screen vast chemical libraries, predict molecular interactions, and identify promising drug candidates with higher precision and efficiency. In addition, AI-powered virtual screening platforms can prioritize compounds for further testing, reducing the number of candidates that need to be evaluated experimentally. AI is also being employed to optimize lead compound structures, enhance drug efficacy, and mitigate toxicity. Furthermore, AI algorithms can predict drug responses in silico, potentially enabling the identification of patient subgroups most likely to benefit from specific therapies [43].
Clinical trial optimization
AI is transforming clinical trial design and execution in hematology, resulting in more efficient and cost-effective drug development. AI algorithms can analyze large patient datasets to identify suitable candidates for clinical trials based on specific inclusion and exclusion criteria. This targeted recruitment strategy can reduce patient heterogeneity and increase the likelihood of detecting meaningful treatment effects. AI can also be used to optimize trial design parameters, such as sample size and endpoint selection, to maximize statistical power and minimize the risk of failure. Additionally, AI-powered platforms can monitor patient adherence and response to therapy in real-time, enabling early detection of adverse events and protocol deviations, thereby safeguarding patient safety and improving trial integrity [44].
Treatment monitoring and response prediction
AI-driven treatment monitoring and response prediction have the potential to transform the management of hematological diseases. AI algorithms can analyze longitudinal patient data, including laboratory results, imaging studies, and wearable device data, to monitor disease progression, detect early signs of relapse, and predict treatment response. This real-time monitoring enables clinicians to adjust treatment strategies promptly, potentially preventing disease progression and improving patient outcomes. Moreover, AI-powered platforms can generate personalized risk scores based on individual patient data, providing valuable insights into disease prognosis and facilitating proactive decision-making [45].
Future Directions and Recommendations
Despite the promising advancements, several challenges need to be addressed to realize the full potential of AI in hematology. A primary concern is the lack of transparency in many AI algorithms, which can hinder interpretability and limit clinician trust. Explainable AI (XAI) is an emerging field that aims to address this issue by developing algorithms that provide transparent and interpretable explanations for their decisions. Additionally, the quality and availability of training data are crucial for the development of robust and generalizable AI models. Bias in training data can lead to biased algorithms, potentially exacerbating healthcare disparities. Ensuring data diversity and representativeness is essential for equitable AI implementation. Furthermore, ethical considerations surrounding AI in hematology cannot be overlooked. Issues such as data privacy, algorithm bias, and the potential for job displacement need to be carefully considered and addressed. The regulatory landscape for AI in healthcare is also evolving, and clear guidelines and standards are necessary to ensure the safety and efficacy of AI-based tools and interventions. Table 2 outlines key ethical considerations, potential challenges, and mitigation strategies associated with AI in hematology.
|
Ethical Consideration |
Potential Challenges |
Mitigation Strategies |
|
Data Privacy and Security |
Unauthorized access or misuse of sensitive patient data |
Robust data governance frameworks, de-identification techniques, and secure data storage and transmission protocols. |
|
Algorithmic Bias |
Bias in training data leading to discriminatory outcomes |
Diverse and representative training datasets, regular audits for bias, and transparency in algorithm development and deployment. |
|
Workforce Impact |
Job displacement or changes in roles for hematologists and laboratory technicians |
Retraining and upskilling programs, focus on tasks requiring human expertise (e.g., complex interpretation, patient interaction). |
|
Regulatory Frameworks |
Evolving landscape for AI in healthcare, challenges in validating and monitoring AI tools |
Clear guidelines and standards for validation, approval, and ongoing monitoring of AI tools, collaboration between regulatory bodies and AI developers. |
The future of AI in hematology is promising, but its successful integration requires a multi-faceted approach. Continued research is needed to develop more robust, interpretable, and generalizable AI models. Collaboration between hematologists, AI researchers, data scientists, and ethicists is crucial to ensure the responsible and equitable development and deployment of AI in hematological practice. Rigorous validation and clinical trials are essential to establish the safety and efficacy of AI-based tools and interventions before their widespread adoption.
Conclusion
AI is set to transform hematology, heralding a new era of unparalleled progress in diagnostic and therapeutic approaches. AI-powered algorithms, leveraging the power of machine learning and deep learning, are transforming the interpretation of blood smears, flow cytometry data, and genomic analyses. These sophisticated tools enhance the accuracy and efficiency of disease detection, classification, and prognostication, ultimately leading to earlier interventions and improved patient outcomes. In the realm of therapeutics, AI is paving the way for personalized medicine, enabling the development of tailored treatment strategies based on individual patient profiles and disease characteristics. AI-driven drug discovery platforms are accelerating the identification of novel therapeutic targets and optimizing clinical trial designs, leading to faster and more cost-effective drug development.
However, as AI continues to permeate hematological practice, it is essential to acknowledge and address the challenges that accompany this technological revolution. The "black box" nature of many AI algorithms raises concerns about transparency and interpretability, hindering clinician trust and hindering the full integration of AI into clinical workflows. Data bias remains a significant hurdle, potentially perpetuating existing disparities in healthcare access and outcomes. Ethical considerations, including patient privacy, data security, and algorithm fairness, must be at the forefront of AI development and deployment.
In conclusion, AI holds immense promise for the future of hematology. By automating routine tasks, improving diagnostic accuracy, and enabling personalized medicine, AI has the potential to transform the way hematological disorders are diagnosed, treated, and managed. However, to fully realize this potential, it is imperative to foster ongoing research, collaboration, and responsible implementation. By addressing the challenges and embracing the opportunities presented by AI, we can pave the way for a new era of precision hematology, where AI-powered tools empower clinicians to deliver optimal care and improve the lives of patients worldwide.
Acknowledgement
The authors extend their sincere gratitude to Prof. Lalima Singh, Principal of SSKGDC and Principal Investigator of the DST-CURIE grant, for her support and encouragement throughout this research endeavor. Additionally, the authors acknowledge with appreciation the financial support provided by the Department of Science and Technology (DST), Government of India, through the DST-CURIE program under sanction number DST/CURIE-PG/2022/10 (G), which was instrumental in facilitating this assignment.
References
2. Andrade CLB, Ferreira MV, Alencar BM, Junior AMA, Lopes TJS, Dos Santos AS, et al. Enhancing diagnostic accuracy of multiple myeloma through ML-driven analysis of hematological slides: new dataset and identification model to support hematologists. Sci Rep. 2024 May 15;14(1):11176.
3. Rösler W, Altenbuchinger M, Baeßler B, Beissbarth T, Beutel G, Bock R, et al. An overview and a roadmap for artificial intelligence in hematology and oncology. J Cancer Res Clin Oncol. 2023 Aug;149(10):7997-8006.
4. Dehkharghanian T, Mu Y, Tizhoosh HR, Campbell CJV. Applied machine learning in hematopathology. Int J Lab Hematol. 2023 Jun;45 Suppl 2:87-94.
5. Bowers KA, Nakashima MO. Digital Imaging and AI Pre-classification in Hematology. Clin Lab Med. 2024 Sep;44(3):397-408.
6. Aslan E, Özüpak Y. Classification of Blood Cells with Convolutional Neural Network Model. Bitlis Eren Üniversitesi Fen Bilimleri Dergisi. 2024;13(1):314-26.
7. Gokulkannan K, Mohanaprakash TA, Beevi LS, Vijayalakshmi R. Leukemia Net: Integrating attention depth wise Separable network-aided stacked feature pooling with weighted recurrent neural network-based leukemia detection model. Biomedical Signal Processing and Control. 2024 Oct 1;96:106459.
8. Lachover-Roth I, Peretz S, Zoabi H, Harel E, Livshits L, Filon D, et al. Support Vector Machine-Based Formula for Detecting Suspected α Thalassemia Carriers: A Path toward Universal Screening. Int J Mol Sci. 2024 Jun 11;25(12):6446.
9. Wan Y, Zhang Y, Li T, Chen S, Niu C, Liao P. Prediction the occurrence of thalassemia with hematological phenotype. J Clin Lab Anal. 2024 Sep 24:e25104.
10. Deif MA, Hammam RE, Solyman AA. Gradient boosting machine based on PSO for prediction of leukemia after a breast cancer diagnosis. International Journal on Advanced Science, Engineering and Information Technology. 2021 Apr 30;11(2):508-15.
11. Elhassan TA, Mohd Rahim MS, Siti Zaiton MH, Swee TT, Alhaj TA, Ali A, et al. Classification of Atypical White Blood Cells in Acute Myeloid Leukemia Using a Two-Stage Hybrid Model Based on Deep Convolutional Autoencoder and Deep Convolutional Neural Network. Diagnostics (Basel). 2023 Jan 5;13(2):196.
12. Barrera K, Merino A, Molina A, Rodellar J. Automatic generation of artificial images of leukocytes and leukemic cells using generative adversarial networks (syntheticcellgan). Comput Methods Programs Biomed. 2023 Feb;229:107314.
13. Mashayekhi H, Nazari M, Jafarinejad F, Meskin N. Deep reinforcement learning-based control of chemo-drug dose in cancer treatment. Comput Methods Programs Biomed. 2024 Jan;243:107884.
14. Mudrik A, Nadkarni GN, Efros O, Glicksberg BS, Klang E, Soffer S. Exploring the role of Large Language Models (LLMs) in hematology: a systematic review of applications, benefits, and limitations. medRxiv. 2024:2024-04.
15. Zhu Y, Ouyang Z, Chen W, Feng R, Chen DZ, Cao J, et al. TGSA: protein-protein association-based twin graph neural networks for drug response prediction with similarity augmentation. Bioinformatics. 2022 Jan 3;38(2):461-8.
16. Gedefaw L, Liu CF, Ip RKL, Tse HF, Yeung MHY, Yip SP, et al. Artificial Intelligence-Assisted Diagnostic Cytology and Genomic Testing for Hematologic Disorders. Cells. 2023 Jun 30;12(13):1755.
17. Chen P, Zhang L, Cao X, Jin X, Chen N, Zhang L, et al. Detection of circulating plasma cells in peripheral blood using deep learning-based morphological analysis. Cancer. 2024 May 15;130(10):1884-93.
18. Bermejo-Peláez D, Rueda Charro S, García Roa M, Trelles-Martínez R, Bobes-Fernández A, Hidalgo Soto M, et al. Digital Microscopy Augmented by Artificial Intelligence to Interpret Bone Marrow Samples for Hematological Diseases. Microsc Microanal. 2024 Mar 7;30(1):151-9.
19. Asghar R, Kumar S, Mahfooz A. Classification of Blood Cells Using Deep Learning Models. arXiv preprint arXiv:2308.06300. 2023 Aug 11.
20. Zhou R, Shu X, Pandey R, inventors; Chinese University of Hong Kong CUHK, assignee. Artificial intelligence enabled reagent-free imaging hematology analyzer. United States patent application US 17/547,033. 2022 Jun 9.
21. Walter W, Pohlkamp C, Meggendorfer M, Nadarajah N, Kern W, Haferlach C, et al. Artificial intelligence in hematological diagnostics: Game changer or gadget?. Blood Reviews. 2023 Mar 1;58:101019.
22. Kimura K, Tabe Y, Ai T, Takehara I, Fukuda H, Takahashi H, et al. A novel automated image analysis system using deep convolutional neural networks can assist to differentiate MDS and AA. Scientific reports. 2019 Sep 16;9(1):13385.
23. Elsabagh AA, Elhadary M, Elsayed B, Elshoeibi AM, Ferih K, Kaddoura R, et al. Artificial intelligence in sickle disease. Blood Reviews. 2023 Sep 1;61:101102.
24. Elemento O. Toward Artificial Intelligence–Driven Pathology Assessment for Hematologic Malignancies. Blood Cancer Discovery. 2021 May 1;2(3):195-7.
25. Radakovich N, Cortese M, Nazha A. Acute myeloid leukemia and artificial intelligence, algorithms and new scores. Best practice & research. Clinical Haematology. 2020 Sep;33(3):101192.
26. Zhou Y, Isozaki A, Yasumoto A, Xiao TH, Yatomi Y, Lei C, et al. Intelligent platelet morphometry. Trends in Biotechnology. 2021 Oct 1;39(10):978-89.
27. Ohsaka A. Artificial intelligence (AI) and hematological diseases: establishment of a peripheral blood convolutional neural network (CNN)-based digital morphology analysis system. [Rinsho ketsueki] The Japanese Journal of Clinical Hematology. 2020 Jan 1;61(5):564-9.
28. Rabbi F, Dabbagh SR, Angin P, Yetisen AK, Tasoglu S. Deep Learning-Enabled Technologies for Bioimage Analysis. Micromachines (Basel). 2022 Feb 6;13(2):260.
29. Pike JA, Simms VA, Smith CW, Morgan NV, Khan AO, Poulter NS, et al. An adaptable analysis workflow for characterization of platelet spreading and morphology. Platelets. 2021 Jan 2;32(1):54-8.
30. Ng DP, Zuromski LM. Augmented Human Intelligence and Automated Diagnosis in Flow Cytometry for Hematologic Malignancies. Am J Clin Pathol. 2021 Mar 15;155(4):597-605.
31. Cheng FM, Lo SC, Lin CC, Lo WJ, Chien SY, Sun TH, et al. Deep learning assists in acute leukemia detection and cell classification via flow cytometry using the acute leukemia orientation tube. Sci Rep. 2024 Apr 9;14(1):8350.
32. Lewis JE, Cooper LAD, Jaye DL, Pozdnyakova O. Automated Deep Learning-Based Diagnosis and Molecular Characterization of Acute Myeloid Leukemia Using Flow Cytometry. Mod Pathol. 2024 Jan;37(1):100373.
33. Monaghan SA, Li JL, Liu YC, Ko MY, Boyiadzis M, Chang TY, et al. A Machine Learning Approach to the Classification of Acute Leukemias and Distinction From Nonneoplastic Cytopenias Using Flow Cytometry Data. Am J Clin Pathol. 2022 Apr 1;157(4):546-53.
34. Fuda F, Chen M, Chen W, Cox A. Artificial intelligence in clinical multiparameter flow cytometry and mass cytometry-key tools and progress. Semin Diagn Pathol. 2023 Mar;40(2):120-8.
35. Kwon R, Yeung CCS. Advances in next-generation sequencing and emerging technologies for hematologic malignancies. Haematologica. 2024 Feb 1;109(2):379-87.
36. Chafai N, Bonizzi L, Botti S, Badaoui B. Emerging applications of machine learning in genomic medicine and healthcare. Crit Rev Clin Lab Sci. 2024 Mar;61(2):140-163.
37. Nazha A, Komrokji R, Meggendorfer M, Jia X, Radakovich N, Shreve J, et al. Personalized Prediction Model to Risk Stratify Patients With Myelodysplastic Syndromes. J Clin Oncol. 2021 Nov 20;39(33):3737-46.
38. Çubukçu HC, Topcu Dİ, Yenice S. Machine learning-based clinical decision support using laboratory data. Clin Chem Lab Med. 2023 Nov 29;62(5):793-823.
39. Kim M, Snowdon J, Weeraratne SD, Felix W, Lim L, Dankwa-Mullan I, et al. Clinical insights for hematological malignancies from an artificial intelligence decision-support tool. J Clin Oncol. 2019;37:e13023.
40. Almutairi NS, Abdulfattah MA, Alqahtani SM, Aljali MA, Almaymoni MH, Almseed YH, et al. Advancements In Hematology: Integrating Precision Medicine Into Clinical Practice. Journal of Namibian Studies: History Politics Culture. 2023 Dec 20;39:103-18.
41. Mohty R, El Hamed R, Brissot E, Bazarbachi A, Mohty M. New drugs before, during, and after hematopoietic stem cell transplantation for patients with acute myeloid leukemia. Haematologica. 2023 Feb 2;108(2):321.
42. Walter W, Pohlkamp C, Meggendorfer M, Nadarajah N, Kern W, Haferlach C, et al. Artificial intelligence in hematological diagnostics: Game changer or gadget?. Blood Reviews. 2023 Mar 1;58:101019.
43. Santos-Silva MA, Sousa N, Sousa JC. Artificial intelligence in routine blood tests. Frontiers in Medical Engineering. 2024 Mar 25;2:1369265.
44. Passamonti F, Corrao G, Castellani G, Mora B, Maggioni G, Gale RP, et al. The future of research in hematology: Integration of conventional studies with real-world data and artificial intelligence. Blood Reviews. 2022 Jul 1;54:100914.
45. Karalis VD. The integration of artificial intelligence into clinical practice. Applied Biosciences. 2024 Jan 1;3(1):14-44.