Keywords
Ac4C, NAT10, KLF6, Ropinirole, Periodontitis, Alveolar bone loss, Inflammatory.
Commentary
Periodontitis is a chronic inflammatory disease that affects more than 40% of the adult population aged over 30 years in the United States, indicating a high prevalence [1]. It impairs the integrity of the tooth-supporting tissue with clinical manifestations featured of gingiva bleeding, periodontal ligament degradation, and alveolar bone resorption. Periodontitis is a multifactorial disease, involving interactions of bacterial pathogens, host immune responses and environmental factors such as smoking [2]. Many studies in the periodontal field indicate that the abnormal immune response is actually excessive inflammatory reactions resulting in the periodontal tissue and alveolar bone destruction [3]. Treatment options for periodontitis can be divided into nonsurgical therapy and periodontal surgical therapy. Periodontal initial therapy is basically nonsurgical, including home care review and scaling and root planing. If active periodontitis still exists, a regenerative or resectional surgical therapy can be received subsequently [2]. Nonsurgical periodontal therapy can mechanically remove dental plaque, whereas targeting only bacteria does not always lead to satisfying outcomes, indicating that current anti-infective therapies of periodontitis have limited efficacy. Thus, it is necessary to seek for breakthroughs from host immune modulation field focused on resolution of inflammation and restoration of tissue homeostasis [4]. Dopamine, a major neurotransmitter, transmits signals via two receptor families: the D1-like receptor (D1R) and the D2-like receptor (D2R). Wang et al. have identified an intracellular secondary signaling pathway for dopamine, revealing its inhibitory role in osteoclast differentiation mediated by the D2R/cAMP/PKA/CREB axis. This study offers new insights into the potential for nervous system’s direct regulation of bone remodeling and supports the clinical potential of dopamine receptor agonists and antagonists in managing bone metabolic disorders [5]. While D2R suppresses osteoclast differentiation, activation of D1R facilitates osteogenic differentiation and reduces bone loss by the ERK1/2 pathway [6]. Moreover, new evidence from a recent study indicates that dopamine exerts its osteogenic effects on periodontal ligament stem cells through both D1 and D2 receptors. This discovery underscores the complexity of dopaminergic signaling in stem cell osteogenesis, as these receptors modulate specific osteogenic markers in distinct ways [7]. Additionally, dopamine derivatives, such as polydopamine, have demonstrated promising applications in bone defect reconstruction and bone regeneration [8,9]. These findings suggest that dopamine receptor agonists and antagonists may have significant therapeutic potential in managing bone metabolic disorders and periodontal diseases. Ropinirole, a D2-like receptor agonist commonly used to treat Parkinson’s disease (PD) and restless legs syndrome, has recently shown potential in treating periodontitis. Isozaki et al. demonstrated that ropinirole suppresses inflammatory cytokine production in gingival tissue through the D2 receptor, thereby reducing neutrophil inflammation and alveolar bone loss in a rat model of periodontitis [10]. Accordingly, ropinirole may be effective in addressing alveolar bone resorption caused by periodontitis. Nevertheless, the precise molecular mechanism underlying these effects remains unclear. Exploring the mechanism from the perspective of epitranscriptomics may reveal novel findings. N4-acetylcytosine (ac4C), acetylation of the N4 position of cytosine, is a post-transcriptional RNA modification catalyzed by N-acetyltransferase 10 (NAT10). Initially discovered in tRNA and rRNA, ac4C modification was later identified on mRNA by Arango et al. in 2018 [11]. ac4C modification in the mRNA coding sequence (CDS) region notably enhances mRNA stability, facilitates mRNA expression, and participates in critical immune and inflammatory signaling in pathogenesis process [12]. NAT10, as the sole confirmed ac4C writer, possesses both acetyltransferase and RNA binding activities [11]. Recently, it was identified that PCBP1/2 and TDP43 function as NAT10 adaptors mediating mRNA acetylation. While NAT10 acts as the core catalytic subunit, PCBP1/2 and TDP43 assist in substrate selection, mRNA binding, and complex stabilization [13]. NAT10 has been implicated in diverse disease processes, including cancer, immune regulation, and viral infections. NAT10 plays an integral role in these disease processes by modulating mRNA stability and translation efficiency of target genes via ac4C modifications, thereby influencing prognosis, cell behavior, stem cell properties, and metabolism [14]. In the context of inflammation, NAT10 has been shown to accelerate lipopolysaccharide (LPS)-induced responses in macrophages through the NOX2-ROS-NF-κB pathway [15]. Besides, NAT10 promotes osteoclast differentiation in inflammatory bone loss by mediating Fos mRNA ac4C modification and upregulating MAPK signaling pathway [16]. Consequently, it can be inferred that NAT10 is crucial to immune modulation and may serve as a potential therapeutic target for managing periodontitis. Given ropinirole’s demonstrated ability to suppress periodontal inflammation and bone resorption, an intriguing question arises: Does ropinirole’s therapeutic effect in periodontitis involve modulation of NAT10 activity? To clarify this question, an in vitro study was carried out to further investigate the pharmacological and molecular mechanism of ropinirole [17]. We measured the inflammatory factor levels of human gingival fibroblasts (HGFs) before and after ropinirole treatment along with the NAT10-mediated ac4C modification regulated by ropinirole. Kruppel-like factor 6 (KLF6), a broadly expressed nuclear transcription regulator, plays a role in regulating cell apoptosis and other physiological process. Served as a direct downstream target of miR-543-3p, KLF6 has been reported to escalate LPS-induced damage in periodontal ligament cells, implying that KLF6 may be a target for periodontitis treatment [18]. Therefore, our study also explored the impact of NAT10-mediated ac4C modification on KLF6. In order to simulate periodontitis, HGFs were treated with LPS. Molecular docking analysis was used to predict the potential binding site between ropinirole and NAT10. The protective effects of ropinirole against LPS-induced injury in HGFs were investigated through assessments of cell viability, cell apoptosis, and inflammatory factor levels. Cell viability was measured using the CCK-8 assay, and cell apoptosis was analysed via flow cytometry. Inflammatory cytokine concentrations (IL-1β, IL-6, and TNF-α) were quantified using ELISA. NAT10 expression and KLF6 mRNA stability were assessed through RT-qPCR, and acRIP-PCR was employed to detect the ac4C modification levels of KLF6 mRNA. The interaction between NAT10 and KLF6 was further confirmed with a dual-luciferase reporter assay. Molecular docking analysis revealed that ropinirole potentially interacts with the catalytic pocket of NAT10, suggesting that this interaction could inhibit its acetyltransferase activity. Our results indicated that LPS significantly reduced cell viability, promoted cell apoptosis and upregulated inflammatory factors, while ropinirole effectively counteracted these negative effects, underlining ropinirole’s protective role in alleviating inflammatory damage. The expression of NAT10 was upregulated by LPS and was downregulated by ropinirole. Overexpression of NAT10 reversed the protective effects of ropinirole on HGFs by inhibiting cell viability, increasing cell apoptosis, and upregulating inflammatory factors. On the contrary, inhibition of NAT10 weakened the effects of LPS on HGFs. The interaction relationship between KLF6 and NAT10 was verified by dual-luciferase reporter assay. NAT10 was shown to increase both the total and ac4C-modified mRNA levels of KLF6 and promote KLF6 mRNA stability. Upregulation of KLF6 reversed the effects of NAT10 inhibition on HGFs. In conclusion, LPS-induced injury in HGFs appears to be associated with NAT10-mediated ac4C modification of KLF6, and ropinirole protects HGFs by inhibiting the NAT10-ac4C-KLF6 axis (Figure 1).
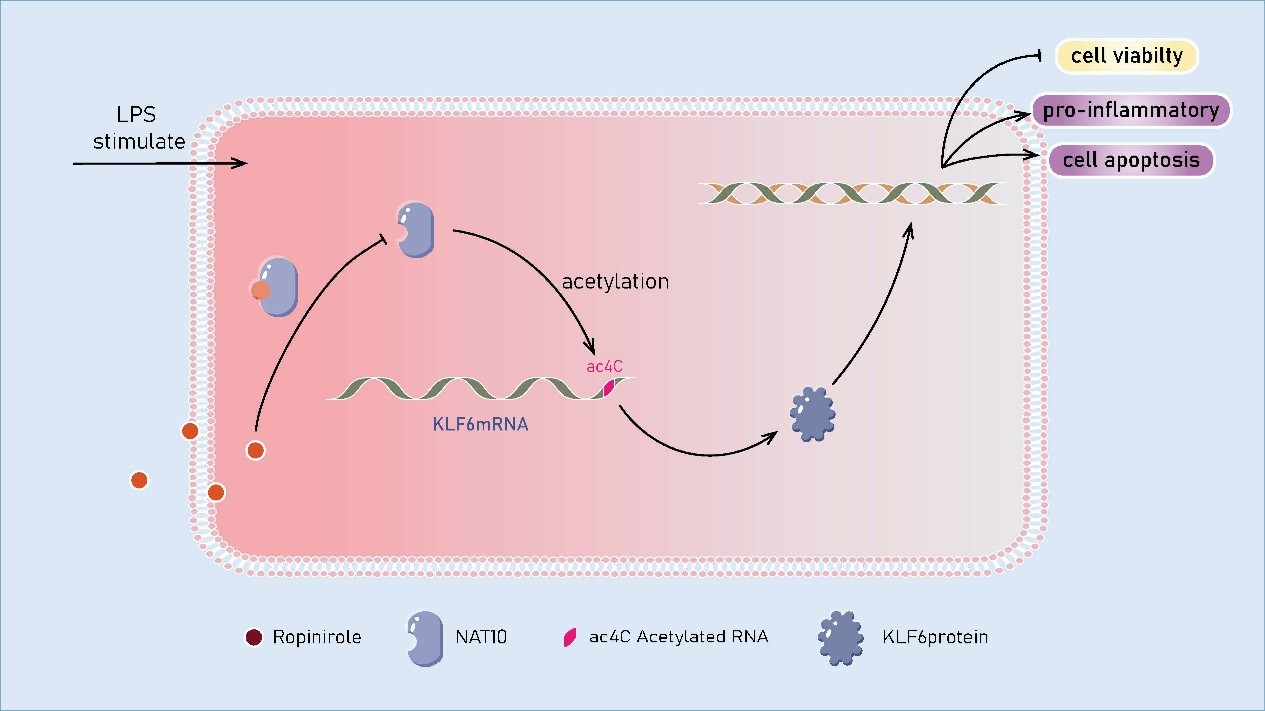
Figure 1. Ropinirole can bind with NAT10, inhibiting its acetyltransferase activity. This inhibition downregulates NAT10 expression and prevents the LPS-induced upregulation of cell apoptosis, reduced cell viability, and inflammatory factors in HGFs. Overexpression of NAT10 reverses these protective effects, while NAT10 inhibition weakens LPS-induced damage. Additionally, NAT10 promotes the expression of KLF6 mRNA through ac4C modification, and upregulation of KLF6 counteracts the effects of NAT10 inhibition. The results suggest that ropinirole protects HGFs by inhibiting the NAT10-ac4C-KLF6 axis.
Our research correlates a drug commonly used for Parkinson’s disease (PD), ropinirole, with the treatment of periodontitis. As a novel inhibitor for NAT10, ropinirole has shown a protective effect against periodontal inflammation through the independent regulation of NAT10-mediated ac4C, suggesting the potential for ropinirole as an adjunct in therapy of periodontitis. In addition, our study highlights targeting NAT10 as a promising therapeutic strategy for managing periodontitis and other inflammation-driven diseases. By advancing the understanding of NAT10’s molecular pathways in oral health, our work paves the way for designing novel therapeutic candidates. Though this study shows promising results, there still remains space for enhancement. Our research unveiled the interrelationship between ropinirole and NAT10, nevertheless, the precise mechanism by which ropinirole interacts with NAT10 on molecular level is not entirely clear, requiring further investigation. On top of that, since the experiments used an in vitro model to simulate periodontitis, animal studies and clinical trials will be a supplement to strengthen the conclusions. A periodontitis rat model with local ropinirole injection can be utilized in animal studies. The ligature-induced periodontitis model is recommended. The main procedure involves placing retentive ligatures, such as silk, nylon, or cotton, around maxillary or mandibular molars under anesthesia [19]. The dosage of ropinirole injection can be referenced from previous in vivo studies. Periodontal destruction and bone resorption could be assessed by micro-CT imaging and HE staining, while expression of inflammatory cytokines could be detected by immunohistochemical staining to further validate our in vitro findings. On the other hand, although ropinirole has a well-established safety profile in Parkinson’s patients, its use in non-Parkinson’s populations, such as those with periodontitis, may raise unique safety concerns. Impulse control disorders (ICDs), including pathological gambling, hypersexuality, and compulsive shopping, are a relatively common side effect of dopamine receptor agonists in patients with PD [20,21]. It is widely acknowledged that dopaminergic medications can lead to addiction in PD, while PD itself does not inherently increase prevalence of ICDs and related disorders [20]. Despite its use in treating PD, ropinirole is also considered as a cause of chronic nausea and vomiting, particularly when used for treating restless legs syndrome [22]. Hence, long-term safety evaluation and monitoring would be necessary in future clinical studies to assess the risk of these side effects in a non-Parkinson’s cohort. Considering the multifactorial nature of periodontitis, it is plausible that other signaling pathways, such as NF-κB and MAPK, may also significantly contribute to its pathogenesis. Future studies could explore the interactions between these pathways and the NAT10-ac4C-KLF6 axis to provide a more comprehensive understanding of the molecular mechanisms involved. In summary, our study reveals that ropinirole, a D2-like receptor agonist, suppresses LPS-induced inflammation in periodontitis by modulating the NAT10-ac4C-KLF6 axis. As a new inhibitor for NAT10, ropinirole has shown a promising protective effect against periodontal inflammation, suggesting the potential for ropinirole as an adjunct in therapy of periodontitis. Moreover, it highlights targeting NAT10 as a hopeful therapeutic strategy for managing periodontitis and other inflammation-driven diseases, advancing the understanding of NAT10’s molecular pathways in oral health and inspiring novel therapeutic candidates. Future in vivo studies are required to validate these findings and assess the broader applications of ropinirole.
Acknowledgement
This work was supported by National Natural Science Foundation of China (No. 82360191); Joint Project on Regional High-Incidence Diseases Research of Guangxi Natural Science Foundation (No. 2023GXNSFBA026125); First-class discipline innovation-driven talent program of Guangxi Medical University.
References
2. Kwon T, Lamster IB, Levin L. Current Concepts in the Management of Periodontitis. Int Dent J. 2021 Dec;71(6):462-76.
3. Loos BG, Van Dyke TE. The role of inflammation and genetics in periodontal disease. Periodontol 2000. 2020 Jun;83(1):26-39.
4. Balta MG, Papathanasiou E, Blix IJ, Van Dyke TE. Host Modulation and Treatment of Periodontal Disease. J Dent Res. 2021 Jul;100(8):798-809.
5. Wang L, Han L, Xue P, Hu X, Wong SW, Deng M, et al. Dopamine suppresses osteoclast differentiation via cAMP/PKA/CREB pathway. Cell Signal. 2021 Feb;78:109847.
6. Zhu J, Feng C, Zhang W, Wang Z, Zhong M, Tang W, et al. Activation of dopamine receptor D1 promotes osteogenic differentiation and reduces glucocorticoid-induced bone loss by upregulating the ERK1/2 signaling pathway. Mol Med. 2022 Feb 21;28(1):23.
7. Sun H, Feng Y, Tu S, Zhou J, Wang Y, Wei J, et al. Dopamine promotes osteogenic differentiation of PDLSCs by activating DRD1 and DRD2 during orthodontic tooth movement via ERK1/2 signaling pathway. Regen Ther. 2024 Apr 8;27:268-78.
8. Wang L, Wu TH, Hu X, Liu J, Wu D, Miguez PA, et al. Biomimetic polydopamine-laced hydroxyapatite collagen material orients osteoclast behavior to an anti-resorptive pattern without compromising osteoclasts' coupling to osteoblasts. Biomater Sci. 2021 Nov 9;9(22):7565-74.
9. Wang L, Hu H, Ko CC. Osteoclast-Driven Polydopamine-to-Dopamine Release: An Upgrade Patch for Polydopamine-Functionalized Tissue Engineering Scaffolds. J Funct Biomater. 2024 Jul 29;15(8):211.
10. Isozaki Y, Sato T, Takagi R, Ito K, Usui M, Kawano M, et al. Ropinirole inhibits inflammatory cytokine production in gingival epithelial cells and suppresses alveolar bone loss in an experimental rat model of periodontitis. Exp Ther Med. 2022 Dec 29;25(2):78.
11. Arango D, Sturgill D, Alhusaini N, Dillman AA, Sweet TJ, Hanson G, et al. Acetylation of Cytidine in mRNA Promotes Translation Efficiency. Cell. 2018 Dec 13;175(7):1872-86.e24.
12. Cui L, Ma R, Cai J, Guo C, Chen Z, Yao L, et al. RNA modifications: importance in immune cell biology and related diseases. Signal Transduct Target Ther. 2022 Sep 22;7(1):334.
13. Jiang ZY, Wu YK, Deng ZQ, Chen L, Zhu YM, Yu YS, et al. PCBP1/2 and TDP43 Function as NAT10 Adaptors to Mediate mRNA ac4C Formation in Mammalian Cells. Adv Sci (Weinh). 2024 Dec;11(47):e2400133.
14. Xie L, Zhong X, Cao W, Liu J, Zu X, Chen L. Mechanisms of NAT10 as ac4C writer in diseases. Mol Ther Nucleic Acids. 2023 Apr 3;32:359-68.
15. Zhang Z, Zhang Y, Cai Y, Li D, He J, Feng Z, et al. NAT10 regulates the LPS-induced inflammatory response via the NOX2-ROS-NF-κB pathway in macrophages. Biochim Biophys Acta Mol Cell Res. 2023 Oct;1870(7):119521.
16. Yang R, Yu W, Lin L, Cui Z, Tang J, Li G, et al. NAT10 promotes osteoclastogenesis in inflammatory bone loss by catalyzing Fos mRNA ac4C modification and upregulating MAPK signaling pathway. J Adv Res. 2024 Jul 31:S2090-1232(24)00318-7.
17. Liao H, Ma H, Meng H, Kang N, Wang L. Ropinirole suppresses LPS-induced periodontal inflammation by inhibiting the NAT10 in an ac4C-dependent manner. BMC Oral Health. 2024 Apr 30;24(1):510.
18. Li W, Wang J, Hao W, Yu C. MicroRNA-543-3p down-regulates inflammation and inhibits periodontitis through KLF6. Biosci Rep. 2021 May 28;41(5):BSR20210138.
19. Rojas C, García MP, Polanco AF, González-Osuna L, Sierra-Cristancho A, Melgar-Rodríguez S, et al. Humanized Mouse Models for the Study of Periodontitis: An Opportunity to Elucidate Unresolved Aspects of Its Immunopathogenesis and Analyze New Immunotherapeutic Strategies. Front Immunol. 2021 Jun 17;12:663328.
20. Carbone F, Djamshidian A. Impulse Control Disorders in Parkinson's Disease: An Overview of Risk Factors, Pathogenesis and Pharmacological Management. CNS Drugs. 2024 Jun;38(6):443-457.
21. Moore TJ, Glenmullen J, Mattison DR. Reports of pathological gambling, hypersexuality, and compulsive shopping associated with dopamine receptor agonist drugs. JAMA Intern Med. 2014 Dec;174(12):1930-3.
22. Kurin M, Bielefeldt K, Levinthal DJ. Prevalence of Nausea and Vomiting in Adults Using Ropinirole: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2018 Mar;63(3):687-93.
