Abstract
Introduction: Stroke and lower extremity venous thromboemboli have been commonly reported following acute infection with severe acute respiratory syndrome of coronavirus-19 (SARS-CoV-2), but spinal cord infarction or ischemia is far less frequent. When diagnosis of either COVID-19 or its neurovascular complications are delayed, functional outcomes may be compromised.
Case presentations: We present 2 middle-aged, Caucasian females with sudden onset of non-traumatic spinal cord injury (NTSCI) due to spinal cord infarction or ischemia attributed to acute infection with SARS-CoV-2. Both demonstrated lower extremity weakness with absent pinprick, but eventually recovered limited ambulation with equipment and bracing. Their reports of myalgias, neuropathic pain, muscle spasticity, and urinary tract complications, including hematuria and infections, exceeded the frequency and severity of other SCI individuals of similar age and degree of neurological impairment. One individual experienced a delay in diagnosis of COVID-19 and the other, a delay in identifying the spinal cord infarction. Both had protracted rehabilitation courses and have since developed post-acute sequelae of SARS-CoV-2 (PASC).
Discussion: Vascular injuries in the form of spinal cord infarction can occur due to SARS-CoV-2, with resultant moderate to severe sensory and motor impairment and long-term disability. Symptoms such as myalgias, neuropathic pain, muscle spasms, and frequent bacterial infections are a part of PASC, independent of any spinal cord injury. The dual presence of PASC and recent SCI attributed to COVID-19 may lead to increased severity of symptoms shared by both conditions, only heightening the need for early rehabilitation.
Keywords
COVID-19, Long COVID, Spinal cord infarction, Non-traumatic spinal cord injury, SARSCoV-2, Rehabilitation, Quality of life
List of Abbreviations
AIDP: Acute Inflammatory Demyelinating Polyneuropathy; ASCIS: Acute Spinal Cord Ischemic Syndrome; CDC: Centers for Disease Control; CSF: Cerebral Spinal Fluid; IC : Intermittent Catheterization; IgG: Immunoglobulin G; IgM: Immunoglobulin M; ISNCSCI: International Standards for Classification of Spinal Cord Injury; LTACH: Long Term Acute Care Hospital; MRI : Magnetic Resonance Imaging; MS : Multiple Sclerosis; NMO: Neuromyelitis Optica; NTSCI: Nontraumatic Spinal Cord Injury; PASC: Post-Acute Sequelae of SARS-CoV-2; PCR: Polymerase Chain Reaction; SARS-CoV-2: Severe Acute Respiratory Syndrome Of Coronavirus-19; SCI : Spinal Cord Injury; TIA: Transient Ischemic Attack; WHO: World Health Organization
Introduction
Spinal cord infarction is extremely uncommon among vascular events, accounting for only 0.3-1% of all strokes and 5-8% of acute myelopathies [1,2]. While deep vein thrombosis, pulmonary embolism, and stroke are commonly observed complications of COVID-19, spinal cord infarction is relatively rare [3-6]. The causes of spinal cord infarction arise from occlusive events in vulnerable areas of the thoracic cord, particularly between T8-12, which is supplied by the artery of Adamkiewicz. The cytokine release, emerging 7 days after contracting SARS-CoV-2, may be responsible for the increase in thrombotic events associated with acute COVID-19 infection. [7,8].
Although more common than SCI infarction, acute spinal cord ischemic syndrome (ASCIS) remains rare among myelopathies, constituting only 5 to 8% of cases [9]. In ASCIS, blood flow has decreased resulting from insufficient oxygen to the cord. ASCIS can be the result of a direct injury to the main blood vessels supplying a given portion of the spinal cord or due to severe systemic hypoperfusion, as in prolonged hypotension from sepsis. The latter has been observed in some cases of severe COVID-19, particularly in situations combined with ARDS, a secondary bacterial sepsis, or a separate cardiac or thrombotic event [10]. In ischemia, blood flow is compromised but not completely interrupted. An infarct is the end result of severe, prolonged ischemia and insufficient oxygen supply, causing total blood flow obstruction and tissue death [11]. Yet, in a substantial number of patients following the process of infarction, partial recovery is observed [12].
The cases discussed in this report differ from other published works describing spinal cord infarcts attributed to acute SARS-CoV-2 due to duration of continuity from initial assessment onward. We have now followed these individuals over 2.5 years after contracting COVID-19, covering their difficulties with “long COVID,” now officially defined named post-acute sequelae of SARS-CoV-2 infection (PASC) by the World Health Organization (WHO) [13]. Each individual in this report had one or more aspects of diagnosis or management delayed, making their cases unique and their acute and long-term recovery all the more challenging.
The term PASC may be assigned to “individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 months from the onset of COVID-19, with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis.” Although fatigue, shortness of breath, and cognitive dysfunction are among the most common complaints in PASC, neurologic symptoms may also occur. By definition, PASC generally impacts everyday functioning. Symptoms may be of new onset, follow initial recovery from an acute COVID-19 episode, or persist from the initial illness. Moreover, symptoms may also fluctuate or relapse over time [13]. While the most common complaints of PASC are fatigue, brain fog or cognitive difficulties, and respiratory concerns, other frequently-cited findings include headache, heart palpitations, exercise intolerance, joint pain or swelling, myalgias, vertigo, peripheral neuropathy, altered taste or smell, disordered sleep, anxiety, depression, and thromboembolic events [14,15]. Although a number of the above symptoms may occur subsequent to SCI, many would be unusual, such as persistent cough, fatigue months after SCI, changes in taste or smell, continued exercise intolerance, and new onset cognitive deficits or “brain fog”, unrelated to any sedating medications.
Our purpose in sharing these cases with readers is: 1) to demonstrate that acute spinal cord infarcts can occur in association with acute COVID-19; 2) to show that onset of both conditions in close proximity may have immediate and lasting effects that appear worse than either condition alone; and 3) to emphasize that integrated medical care and customized rehabilitation for both conditions must be provided as early as possible to optimize functional improvement. Both individuals became symptomatic prior to COVID-19 vaccine availability and have given written consent to share their stories for educational publication. This project was approved by the Institutional Review Board of the MetroHealth System.
Case 1
A 40-year old Caucasian female developed an acute viral illness during a 2-week transatlantic cruise carrying 4,500 persons in February 2020. Initial symptoms included high fever above 102°F, myalgias, cough, pharyngitis, and fatigue. She was traveling with relatives who became similarly ill, all of whom were informed by cruise administration that a “viral syndrome” was present on the ship. The patient stated that hundreds of other passengers were ill, including her cabin mates, travel partners, and some from other countries with whom she had dined. Although hand sanitizer was instituted broadly, communal buffet style dining was maintained and isolation to cabins for those ill was voluntary. Passengers on the ship were from Western Europe, including Spain and Italy, as well as China, all areas endemic to COVID-19 in February 2020. Other travelers were from the United States and Canada. People entered and exited at various ports. No COVID-19 testing was available on board and no masks were dispensed. Influenza testing was negative, and the cruise-based clinicians recommended acetaminophen, occasional ibuprofen, and non-prescription cough suppressants. No medications typically given for acute COVID were offered. No methods of preventing infection dissemination were undertaken because the staff may not have recognized the illness as COVID-19. Some travelers whom she befriended and dined with have since developed ongoing non-neurologic symptoms common to PASC, and one individual she came to know well passed away two months after the trip. This cruise ship has been under investigative review by the Centers for Disease Control (CDC) due to substantial numbers of identified cases on board, diagnosed subsequent to the journey.
Upon arriving home, she remained incapacitated for several additional days due to illness but then returned to work. Ten days after reaching home and 14 days after the start of the viral illness, she had acute back pain followed an hour later by rapid onset of sensory and motor loss in the subsequent 75 minutes. Symptoms continued to worsen, thus she presented to the emergency department with severe pounding back pain, tightness in her legs, complete absence of motor function in both lower extremities, and a profound sensory loss at the T6 dermatome with only minimal light touch preservation below this level. She was unable to urinate and required bladder catheterization for over 1 liter upon arrival at the hospital. Although she continued to have flu-like symptoms, viral titers for Influenza, Epstein-Barr, Herpes Simplex, and West Nile viruses were negative. Inflammatory markers of anti-Aquaporin-4, and anti-myelin oligodendrocyte glycoprotein were also negative. Her full diagnostic work up is given in Table 1. Acute Inflammatory Demyelinating Polyneuropathy (AIDP) was considered, but further investigation was deferred, based on presence of a defined sensory level, acute sensorimotor loss within 2 hours, and imaging findings. She did not demonstrate albuminocytologic dissociation, a finding characteristic of AIDP [18,19]. Based on the preceding data, an electromyographic investigation to diagnose AIDP was not performed in the acute setting but instead was done 11 months later for a different purpose. Results showed normal distal motor latencies and conduction velocities of tibial and peroneal nerves. No conduction block was found, but decreased amplitude of compound muscle action potentials was noted. COVID-19 was not considered because she became ill in mid-February 2020, prior to widespread recognition of SARS-CoV-2 in the Midwestern United States. She did not know more than that she had experienced a viral illness on the cruise at that time.
|
Lab |
Case 1 |
Case 2 |
|
SARs COV 2 |
NT |
positive |
|
Influenza A and B |
negative |
negative |
|
CSF enterovirus |
NT |
NT |
|
CSF VZV |
Not detected |
Not detected |
|
CSF WNV |
Not detected |
Not detected |
|
CSF HSV |
Not detected |
Not detected |
|
AQP4 |
Not detected |
Not detected |
|
Myelin basic protein |
NT |
NT |
|
MOG ab, IgG |
negative |
negative |
|
Oligoclonal bands |
Not present |
Not present |
|
IgG synthesis CSF and serum |
WNL |
NT |
|
HIV Ab-Ab |
nonreactive |
NT |
|
VDRL CSF |
nonreactive |
NT |
|
Lupus anticoagulant |
negative |
negative |
|
Anti-cardiolipin IgG, IgM |
WNL |
NT |
|
Beta2glycoprotein IgG, IgM, IgA |
WNL |
NT |
|
Lyme Antibodies IgG, IgM |
negative |
negative |
|
Paraneoplastic panel |
negative |
NT |
|
This table gives the laboratory tests completed on each case. NT: Not Tested; CSF: Cerebrospinal Fluid; WNL: Within Normal Limits; VZV: Varicella Zoster Virus; WNV: West Nile Virus; HSV: Herpes Simplex Virus; AQP4: Aquaporin-4; MOG: Myeline Oligodendrocyte Glycoprotein; ab: antibody; IgG: Immunoglobulin G; HIV: Human Immunodeficiency Virus; VDLR: Venereal Disease Research Laboratory; IgM: Immunoglobulin M; IgA: Immunoglobulin A. |
||
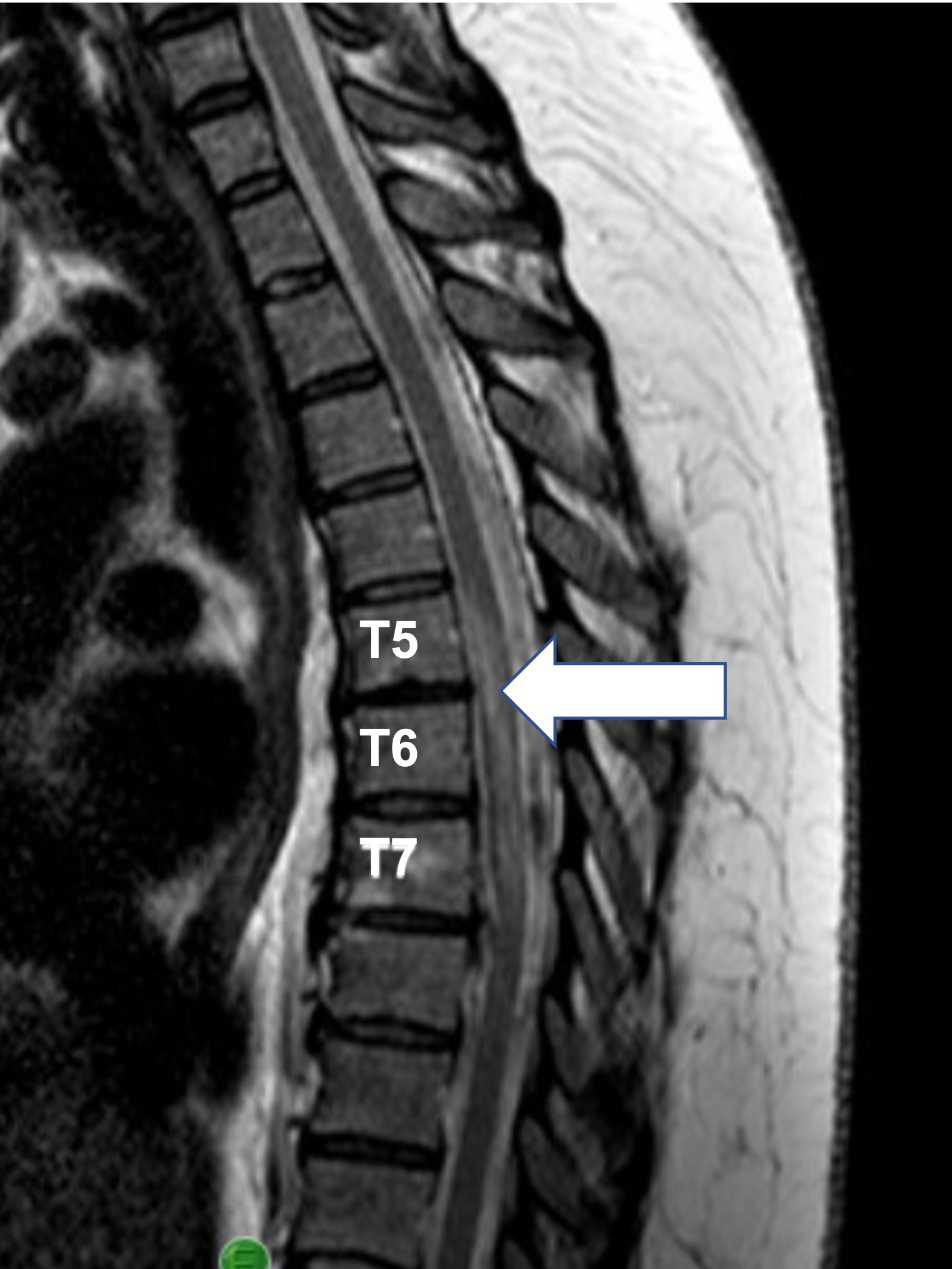
Her initial MRI one day after presentation was unremarkable, but on the following day, imaging demonstrated T2 hyperintensity from T5-7 (Figure 1). She was given 5 days of IV methylprednisolone but not intravenous immunoglobulin or plasmapheresis. At the time of acute care discharge, her diagnosis was not fully determined, with the differential being spinal cord infarct versus a demyelinating disease other than multiple sclerosis (MS) or neuromyelitis optica (NMO). Although ultimately the cause was identified as a spinal cord infarct, the debate in diagnosis caused delayed initiation of comprehensive SCI rehabilitation, occurring approximately 18 days following the onset of paraplegia.
Figure 1. An acute T2 intramedullary signal hyperintensity is seen T5-T7, consistent with spinal cord infarct vs demyelinating disease. Clinical presentation and laboratory findings strongly suggested a diagnosis of infarct over demyelinating disease.
It was during her inpatient rehab stay that the possibility of COVID-19 became clear. Unfortunately, she was beyond the window for polymerase chain reaction (PCR) testing and antibody testing was not available. This patient meets the definition of probable COVID-19 based on direct contact with a probable case (in her situation, more than one case); her linkage to a cluster, and meeting clinical criteria of a probable case [20]. Her initial rehabilitation course for incomplete paraplegia was notable for escalating neuropathic pain, myalgias, spasticity, and urinary retention. By the end of inpatient rehabilitation, the weakness improved with therapy and bowel program had reestablished regularity of stool evacuation. However, a nagging cough, myalgias, spasticity, and fatigue remained.
In her first year of outpatient care, the neuropathic pain was severe but eventually able to be managed with anti-seizure medications. Urinary tract infections were frequent and urinary retention was present for the first several months. The infections prompted 5 urgent care visits, but improved over subsequent months as did her ability to evacuate. What remained unmanageable among neurological symptoms was the lower limb spasticity.
After 18 months of failed quarterly Botulinum toxin treatments and oral medications for spasticity, an intrathecal baclofen pump was placed, which became repeatedly infected leading to its explantation. This individual now has an equinovarus contracture of her left foot and ankle from unremitting tone. Tendon releases and surgical reconstruction were then undertaken to aid with ambulation in the presence of partial motor recovery. Continued outpatient physical therapy is helping her regain ambulation. Additional symptoms specific to PASC in this patient are given in Table 2. These include ongoing fatigue for over 2 years; an intermittent “hacking” cough; brain fog in the form of concentration lapses and mild short-term memory deficits; headache; and chronic myalgias, both above and below the region of NTSCI, all atypical of spinal cord disorders but contributing to depression and reduced quality of life. Neuropathic pain and spasticity agents were used in varying amounts over the prior 3 years for her discomfort. Yet, the attempted reduction of such medication did not improve cognitive symptoms or fatigue, but only worsened her pain and myalgias. A combination of outpatient speech therapy and use of memory logs, reminder signals on her smart phone, and other assistive technology allowed her to slowly return to work. Her endurance and stamina have never recovered resulting in deliberate energy conservation measures throughout the day. More than 36 months after losing function, she still has no pinprick below T7 and demonstrates significant weakness in both legs.
|
Symptoms |
Sneller et al. [31] |
Davis et al. [29] |
Case 1 |
Case 2 |
|
Fatigue |
26% |
98.3% |
Yes |
Yes |
|
Cough |
5% |
66.2% |
Yes |
Yea |
|
Concentration Deficit |
12% |
85.1% |
Yes |
Yes |
|
Dyspnea |
19% |
77.4% |
Yes |
Yes |
|
Anosmia/parosmia |
14% |
35.9% |
Yes |
No |
|
Headache |
12% |
77% |
Yes |
Yes |
|
Insomnia |
9% |
60% |
Yes |
Yes |
|
Chest pain/discomfort |
8% |
53.1% |
No |
No |
|
Anxiety |
6% |
57.9% |
Yes |
Yes |
|
Myalgia |
6% |
69.1% |
Yes |
Yes |
|
Tinnitus |
6% |
26.2% |
No |
No |
|
Palpitations |
5% |
67.4% |
Yes |
Yes |
|
Arthralgia |
3% |
52.2% |
Yes |
Yes |
|
Taste Disorder |
5% |
33.7% |
Yes |
Yes |
|
Depression |
3% |
47.3% |
Yes |
Yes |
|
Alopecia |
4% |
N/A |
No |
Yes |
|
Dizziness |
4% |
67.3% |
No |
Yes |
|
Paresthesias |
1% |
35.4% |
Yes |
Yes |
|
Visual Impairment |
1% |
10.4% |
Yes |
Yes |
|
Symptoms of two large COVID studies compared with the same symptoms in our case presentations, occurring at any point beyond 90 days of diagnosis. |
||||
Case 2
A 52-year-old Caucasian female presented to acute care in late November 2020 with acute hypoxic respiratory failure and was diagnosed with COVID-19 pneumonia. Due to severe pulmonary edema and inability to maintain adequate oxygen saturations, she was intubated and placed on mechanical ventilation. She had been able to communicate with writing and was responsive, despite respiratory failure. Although she reported weakness, she was moving 4 limbs antigravity at that time per report. On hospital day 26, she experienced an asystolic cardiac arrest with return of spontaneous circulation after 2 cycles. During this time, she was hypotensive with systolic blood pressure in the 60’s and had limited alertness. It was not known how hypotensive she may have been in the hours prior to the arrest.
CT of the brain raised suspicion for a small subarachnoid hemorrhage in the left frontal lobe with no mass effect, and normalization of imaging findings on subsequent study 12 days later. No spine imaging was done as part of resuscitation work up. On hospital day 35, fevers arose without identified infection in blood, urine, or lung, prompting further procedures and testing. However, analysis of findings revealed no obvious inflammatory or new infectious cause (Table 1) of either fever or weakness. During this time, no physician performed an evaluation of her sensorimotor loss by examination using International Standards for Classification of Spinal Cord Injury (ISNCSCI). She was able to communicate via writing, mouthing words silently, and gestures, but was still unable to verbally communicate while on the ventilator at that time. Two days later, the patient was transferred to long term acute care hospital (LTACH) for continued vent weaning and severe weakness, attributed to critical illness in the chart.
The patient’s motor weakness became more obvious at the LTACH. Although she had reported generalized weakness before her cardiac arrest, she believes she was not able to move “anything” in her legs after the cardiac event. At the LTACH, she subsequently endorsed trace movement in her lower extremities as well as new paresthesia in the lower trunk, legs, and sacral areas. The referring hospital listed “critical illness polyneuropathy” among their discharge diagnoses. After successful vent weaning following 14 days at the LTACH and 54 days since confirmed diagnosis with COVID-19, this individual was admitted to inpatient rehabilitation with a presumed diagnosis of critical illness polyneuropathy.
Clinical exam at that time was notable for inability to urinate, requiring intermittent catheterization (IC); neurogenic bowel; profound orthostatic hypotension without tachycardia; lower extremity paraplegia, and hyperreflexia. While her initial sensory exam showed deficits to light touch beginning at the L2 dermatome, a later exam found deficits in pinprick distal to T9, a finding that has persisted for 30 subsequent months.
Due to anxiety and pain, she was unable to tolerate a full MRI in rehab, but no clear infarct was found from the images completed. She was given a revised potential diagnosis of ASCIS based on history and exam showing sensory, motor, and autonomic deficits consistent with NTSCI.
During the first year, she received monthly intravesicular injections of anesthetics due to searing but non-spastic bladder pain that limits daily activities. Hematuria continued even after stopping IC and recovering ability to void. This individual did continue to have bladder spasms, in the absence of infethisn, but beta-3 agonists were helpful. Due to her ongoing hematuria, anticoagulation was stopped. One month after discharge from inpatient rehabilitation, she sustained a mild right-sided stroke that resulted in no further loss of motor function. However, sensory changes were found in her left arm and left upper trunk, regions which had demonstrated normal sensation by the end of inpatient rehabilitation. Imaging showed no intracerebral changes. The event was officially classified as a transient ischemic attack but worsened her left shoulder pain. Nonetheless this additional ischemic event supports a repeated pattern of vascular events, this time without a provoked illness contributing to causation. In addition, headaches and dizziness have persisted and may be attributed to post-stroke symptoms plus possible contribution from PASC. She has regained partial household ambulation with the combined use of one short-leg brace and a wheeled walker. She continues to demonstrate reduced sensation with bilateral absence of pinprick below the T9 dermatome. During her second year after acute COVID-19, she developed rotator cuff tendinopathy, precipitated by heavy reliance of assistive devices for functional mobility.
Difficulties with cognition, attention and concentration were evident during inpatient rehabilitation. The TIA that occurred a month after discharge from inpatient did not worsen concentration or attention. However, the use of memory strategies, reminder alarms, planners, and other means of organizing her thoughts were insufficient to allow independent living. After several months of day-long family supervision and failed nonpharmacologic strategies, amantadine was initiated to mitigate the cognitive and concentration lapses associated with COVID-19. This combination of pharmacologic and physiatric interventions eventually permitted transition to a residential group home [16,17].
More than 2.5 years after diagnosis with COVID-19, she continues to experience unremitting neuropathic pain and spasms, with symptoms just as intense as they were at the onset of COVID-19. Throughout the last 2.5 years, symptoms of PASC have also included fatigue, poor exercise tolerance, short term memory and neurocognitive deficits, depression, insomnia, and headaches. These have rendered her unable to work or live without the support of others. As with Case 1, frequent urinary tract infections remain disruptive to her quality of life even if the bladder pain and hematuria have lessened.
Discussion
Each case described in this narrative is characterized by uncertainty with particular features of the diagnosis and by the unpredictability of the clinical course. Both individuals described significant neuropathic pain, spasms, and bladder symptoms, yet bowel issues were infrequent and pressure injuries absent.
In Case 1, spinal cord involvement along with a clinical diagnosis of COVID-19 had been determined by the end of her rehabilitation, although the viral association and causality had been unclear upon admission to rehabilitation and during acute care. Initially, there had been some debate as to whether the NTSCI was infarction or demyelination. The presence of abrupt onset of back pain, lesions in key areas of ischemia and infarction, rapid motor loss with areflexia and negative CSF studies are strongly suggestive of a vascular injury. Moreover, the timing of motor and sensory loss was 2-3 hours. A spinal cord infarction results in rapid sensory and motor loss over minutes to a few hours, but not over the course of a full day or longer. Pain at the level of the lesion and its adjacent areas, just preceding the loss of motor function or simultaneous with it is common [18,19]. While MRI findings on T2-weighted sequences may not be revealing immediately, 8-72 hours after the onset of an infarct, a hyperintensity involving the centromedullary region with sparing of the anterior rim and encompassing a minimum of 3 vertebral segments on sagittal views, becomes evident [18]. An exhaustive lab workup refuted alternative diagnoses, but these measures delayed other aspects of care, including elimination of causes of vascular injury and timely transfer to spinal cord inpatient rehabilitation.
This case was particularly notable for the degree of spasticity the individual experienced and the heroic measures needed to treat her continued tone, including baclofen pump implantation. Moreover, the number of infections in both the intrathecal pump and on other occasions in the urinary tract, is at the upper range of what is typically observed in an incomplete (ASIA impairment C) SCI individual, particularly given that she has been able to fully evacuate her bladder since the time of discharge from inpatient rehabilitation. It remains unclear if her course would have been less severe had she been diagnosed with SARS-CoV-2 at the onset of the acute respiratory symptoms and had received antivirals at the time of presentation. The IV methylprednisolone she received was for a possible inflammatory etiology of the SCI, not weeks earlier at the time of SARS-CoV-2 onset.
Our second case was notable for lack of a timely, formal work up involving spinal cord pathology and for its diagnostic challenges related to the delay. It was not until the patient came to inpatient rehabilitation where her symptoms of profound weakness, sensory loss, orthostatic hypotension, and neurogenic bowel and bladder, in the form of retention rather than incontinence, were indicative of spinal cord dysfunction. History would suggest that a central neurologic illness within the spinal cord is more likely than any form of peripheral neuropathy. Her acute cardiac arrest and systemic hypotension (which was not immediately corrected) could have led to a watershed injury in mid-thoracic cord. The hypotensive crises and cardiac arrest were due to critical illness caused by SARS-CoV-2. In this case, ASCIS is a secondary consequence of the virus and its downstream effects, rather than a more direct cause for an infarct as in Case 1.
Unlike a distinct cord infarct, the diagnosis of ASCIS can escape detection on conventional MRI, further adding to the diagnostic challenge [20,21]. Diffusion–weighted imaging (DWI) is best for early detection of both ischemia and infarct between 3 hours and 11 days after an identified event [22,23]. However, in some cases of more transient ASCIS, findings may never appear on MRI, although would on a well-timed DWI. Cost and availability of this newer imaging modality can be prohibitive [21,24]. No suggestion of transverse myelitis or NMO was present, and earlier inflammatory markers and infectious indices were negative. Given the development of an ischemic stroke after leaving rehab, her propensity to develop ischemia is apparent. This tendency in combination with truncal sensory findings further support a diagnosis of lower thoracic cord infarct, rather than a peripheral polyneuropathy associated with critical illness.
The differential diagnoses of spinal cord infarct after a viral illness includes acute inflammatory demyelination polyneuropathy (AIDP); idiopathic transverse myelitis (ITM) or viral myelitis; MS; NMO; sudden cord compression from a neoplastic, vascular, or degenerative process. Diagnosis of a spinal cord infarct also involves the elimination of other conditions that can mimic this disorder, clinically or radiographically. The absence of demyelinating lesions in the brain eliminated the possibility of MS in cases 2 and 3 [25]. The presence of spinal enhancement in Case 1 ruled out AIDP. The onset of weakness following a hypotensive episode along with bowel and bladder abnormalities render acute inflammatory demyelinating polyneuropathy (AIDP) unlikely in Case 2. The presence of hyperreflexia upon admission to rehab further points to a diagnosis other than AIDP. An electromyogram was ordered as an outpatient but was cancelled when the patient had a second cerebrovascular event, lending support to an ischemic etiology for her initial condition. No exam findings of optic neuritis were seen and AQP4 was non-detectable, rendering a diagnosis of NMO unlikely [26]. CSF findings of pleocytosis or elevated IgG are often found in individuals with ITM but were not observed in either of our patients [27].
Both individuals presented in this report underwent numerous interventions to manage their intense symptoms, and more than 2.5 years later, a number of their medical issues remain unchanged. These struggles are similarly echoed by many of those with PASC who endure daily challenges that severely impact their quality of life and ability to participate in the community [28]. The individual in Case 1 represents one of the many thousands of persons who have PASC symptoms in the absence of a positive COVID-19 laboratory test or antibody confirmation. She experienced COVID-19 before tests were widely available and even prior to national recognition of its eventual magnitude. In early 2020, PCR testing was limited, and when performed, was often done too late to capture positivity. In a study of 3,762 persons identified with long COVID, only 27% had a positive COVID PCR antigen test or a positive SARS-CoV-2 IgG or IgM antibody [29]. This result was due to lack of available testing (48.3%) or delayed testing resulting in a negative finding (24.5%).
In a case series of those with NTSCI directly attributed to COVID-19, consisting of individuals with spinal cord infarction, ITM, or NMO, the most persistent complaints were myalgia, neuropathic pain, and bladder dysfunction in the forms of bladder spasms, hematuria, and urinary tract infections [30]. While myalgias and neuropathic pain are commonly reported, the urinary symptoms of PASC, apart from renal impairment, have only recently been recognized [29,31]. Lamb and colleagues found a high rate of COVID-19-associated cystitis among those with PASC [32]. Their group has also linked COVID-19 inflammation to an increase in urine cytokines and to bladder hyperreflexia, nocturia, and urge incontinence [33]. In a person with SCI, such factors could certainly contribute to frequent urinary tract infections. The individual in Case 2 had prolonged hematuria even after cessation of IC. Although she was on prophylactic dose anticoagulation, inflammatory factors may have contributed to such prolonged hematuria.
The importance of rehabilitation is evident in both cases, yet each faced one or more delays in diagnosis or management, prolonging their transition to rehabilitation. In the first case, lack of resources prevented timely diagnosis of COVID-19, and absence of treatment of her early viral symptoms likely contributed to her fatigue and to her reduced pulmonary and physical endurance. The exhaustive exploration of alternative diagnoses to spinal cord infarct meant that intensive physical, occupational, and pulmonary rehabilitation did not begin until several weeks after acute sensorimotor loss. After diagnostic testing and specialized medication delivery had concluded, the remaining time spent waiting for test results took place in acute care where therapy was very limited. Instead, this time could have been more meaningfully spent on the rehabilitation unit.
Transition to focused spinal cord rehabilitation in a specialized center, geared toward the needs of acute SCI patients should occur as early as possible to optimize functional outcomes [34]. However, transitions to inpatient SCI rehabilitation occurring within 29 days, as it was in this case, may have no ultimate impact on outcomes. However, special efforts and additional resources are needed for more vulnerable populations or those with unusual needs [35]. The authors believe individuals with acute spinal cord infarction attributed to acute infection with SARS-CoV-2 warrant special consideration for early transfer to rehabilitation. Although the above investigations studied traumatic SCI, their findings are applicable to many of those with NTSCI.
Delays in admission to acute rehabilitation were even more pronounced in our second patient. In her case, one significant problem was the very late identification of functional impairment to her legs, from the day of the ischemic episode, through her time in intensive care when a full ISNCSCI was clinically indicated, to her time in the LTACH when she was more fully alert and able to speak. Earlier recognition of the NTSCI might have improved her long-term prognosis. Despite the prolonged wait to begin acute SCI rehabilitation, COVID-specific therapy measures for speech, breathing endurance, muscle aches, and energy conservation were combined with education about SCI clinical sequelae. This approach, incorporating both COVID rehabilitation measures and diagnosis-specific neurologic rehabilitation therapies, is feasible, safe, and effective, leading to improved functional outcomes [36]. The 2 individuals presented here have combined disabilities from SCI and now PASC, making their long-term prognosis more difficult. A recent report from the United States Department of Health and Human Services indicates these individuals fall into the age groups with the two highest rates of PASC [37]. Close follow up in the outpatient setting will be essential to sustain functional progress, manage other neurologic sequelae of PASC that may arise [38], and maintain or improve current quality of life.
Conclusion
In both cases described in this report, the neurological sequelae of COVID-19 may be contributing to the secondary effects of NTSCI. In the chronic care setting, physicians may have difficulty separating which symptoms are due to a NTSCI and which are a direct consequence of PASC. Given the devastating combined effect of SCI and PASC on a person’s quality of life, appropriate rehabilitation interventions for each condition must be developed, regardless of the cause. One lingering question remains whether PASC is more severe in individuals whose SCI was attributed to COVID-19 than it is in those who contracted COVID-19 shortly after a new traumatic SCI or a chronic SCI. Certainly, the cases here illustrate the ongoing struggles and unfavorable outcomes of NTSCI, directly or indirectly attributed to COVID-19. Over the coming years, the spinal cord community of research scientists and clinicians will learn more about such issues. Large numbers of persons living with SCI could face a new disability. It will be our role to provide life-long care for their ongoing COVID-19 concerns, as well as their chronic spinal cord injury needs.
Ethical Approval and Consent To Participate
This case series is a portion of a larger project approved by the MetroHealth System Institutional Review Board. Because this case series involved individuals with their clinical details, separate written consents using IRB approved forms for publication of case reports were obtained from each participant.
Consent for Publication
Written informed consent was provided by all participants.
Author Contributions
CVO was responsible for concept design, for the great majority of the writing of this manuscript, for obtaining patient consent, and for portions of data extraction. SS was responsible for background information, literature review, and formatting of the manuscript including retain and formatting of tables and reference list. ACO was responsible for background information, obtaining periodic clinical updates from the persons in this report, and portions of the literature review and image extraction and translation to jpg format. All authors approved the version to be published.
Acknowledgements
The authors wish to acknowledge the effort of Samuel Onusko MD in creating a secure database for entry of demographic and clinical data, and categorizing that data for analysis.
Funding
There was no specific funding for this project.
Competing Interests
The authors have no competing interests that would be relevant for this publication.
Availability of Data and Materials
Due to the personal nature of these case reports, additional data are unable to be shared with other researchers. However, inquiries of any nature can be forwarded to the corresponding author and responses will be sent within the permitted constraints of the Institutional Review Board of the MetroHealth System.
References
2. Novy J, Carruzzo A, Maeder P, Bogousslavsky J. Spinal cord ischemia: clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Archives of Neurology. 2006 Aug 1;63(8):1113-20.
3. Sampogna G, Tessitore N, Bianconi T, Leo A, Zarbo M, Montanari E, et al. Spinal cord dysfunction after COVID-19 infection. Spinal Cord Series and Cases. 2020 Sep 30;6(1):92.
4. Eissa M, Abdelhady M, Alqatami H, Salem K, Own A, El Beltagi AH. Spinal cord infarction in a 41-year-old male patient with COVID-19. The Neuroradiology Journal. 2021 Jun;34(3):245-8.
5. Bax F, Gigli GL, Iaiza F, Valente M. Spontaneous spinal cord ischemia during COVID-19 infection. Journal of Neurology. 2021 Nov;268(11):4000-1.
6. Kahan J, Gibson CJ, Strauss SB, Bronstein M, Winchell RJ, Barie PS, et al. Cervical spinal cord infarction associated with coronavirus infectious disease (COVID)-19. Journal of Clinical Neuroscience. 2021 May 1;87:89-91.
7. Zhang S, Zhang J, Wang C, Chen X, Zhao X, Jing H, et al. COVID-19 and ischemic stroke: Mechanisms of hypercoagulability. International Journal of Molecular Medicine. 2021 Mar 1;47(3):1.
8. Coperchini F, Chiovato L, Croce L, Magri F, Rotondi M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine & Growth Factor Reviews. 2020 Jun 1;53:25-32.
9. Nedeltchev K, Loher TJ, Stepper F, Arnold M, Schroth G, Mattle HP, et al. Long-term outcome of acute spinal cord ischemia syndrome. Stroke. 2004 Feb 1;35(2):560-5.
10. Hanidziar D, Bittner EA. Hypotension, Systemic Inflammatory Response Syndrome, and Coronavirus Disease 2019: A Clinical Conundrum. Anesthesia and Analgesia. 2020 Jun 6;131(3):e175-e176.
11. Stedman TL. Stedman’s Medical Dictionary. 28th Edition. Philadelphia: Lippincott Williams & Wilkins; 2006.
12. Robertson CE, Brown RD, Wijdicks EF, Rabinstein AA. Recovery after spinal cord infarcts: long-term outcome in 115 patients. Neurology. 2012 Jan 10;78(2):114-21.
13. WHO A. clinical case definition of post COVID-19 condition by a Delphi consensus. https://www. who. int/publications/i/item. WHO-2019-nCoV-Post_COVID-19_Condition-Clinical_Case_Definition-2021.16 October. 2021.
14. Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nature Medicine. 2021 Apr;27(4):601-15.
15. Bull-Otterson L, Baca S, Saydah S, Boehmer TK, Adjei S, Gray S, et al. Post–COVID conditions among adult COVID-19 survivors aged 18–64 and≥ 65 years—United States, March 2020–November 2021. Morbidity and Mortality Weekly Report. 2022 May 5;71(21):713-7.
16. Sami MB, Faruqui R. The effectiveness of dopamine agonists for treatment of neuropsychiatric symptoms post brain injury and stroke. Acta Neuropsychiatrica. 2015 Dec;27(6):317-26.
17. Greenspan RD, Oleson CV. The value of amantadine to improve brain fog in post-acute sequelae of SARS-CoV-2. American Journal of Physical Medicine and Rehabilitation. 2023 Apr;102(4S):A210.
18. Boddu SR, Cianfoni A, Kim KW, Banihashemi MA, Pravatà E, Gobin YP, et al. Spinal cord infarction and differential diagnosis. In: Saba L, Raz E, Editors. Neurovascular Imaging: From Basics to Advanced Concepts. New York: Springer; 2014. p. 1-64.
19. Masson C, Pruvo JP, Meder JF, Cordonnier C, Touzé E, De La Sayette V, et al. Spinal cord infarction: clinical and magnetic resonance imaging findings and short term outcome. Journal of Neurology, Neurosurgery & Psychiatry. 2004 Oct 1;75(10):1431-5.
20. Zalewski NL, Rabinstein AA, Krecke KN, Brown RD, Wijdicks EF, Weinshenker BG, et al. Characteristics of spontaneous spinal cord infarction and proposed diagnostic criteria. JAMA Neurology. 2019 Jan 1;76(1):56-63.
21. Alblas CL, Bouvy WH, Lycklama a Nijeholt GJ, Boiten J. Acute spinal-cord ischemia: evolution of MRI findings. Journal of Clinical Neurology. 2012 Sep;8(3):218-23.
22. Thurnher MM, Bammer R. Diffusion-weighted MR imaging (DWI) in spinal cord ischemia. Neuroradiology. 2006 Nov;48:795-801.
23. Loher TJ, Bassetti CL, Lövblad KO, Stepper FP, Sturzenegger M, Kiefer C, et al. Diffusion-weighted MRI in acute spinal cord ischaemia. Neuroradiology. 2003 Aug;45:557-61.
24. Zhang JS, Huan Y. Multishot diffusion-weighted MR imaging features in acute trauma of spinal cord. European Radiology. 2014 Mar;24:685-92.
25. Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. The Lancet Neurology. 2018 Feb 1;17(2):162-73.
26. Lalan S, Khan M, Schlakman B, Penman A, Gatlin J, Herndon R. Differentiation of neuromyelitis optica from multiple sclerosis on spinal magnetic resonance imaging. International Journal of MS Care. 2012 Dec 1;14(4):209-14.
27. Nance JR, Golomb MR. Ischemic spinal cord infarction in children without vertebral fracture. Pediatric Neurology. 2007 Apr 1;36(4):209-16.
28. Tabacof L, Tosto-Mancuso J, Wood J, Cortes M, Kontorovich A, McCarthy D, et al. Post-acute COVID-19 syndrome negatively impacts physical function, cognitive function, health-related quality of life, and participation. American Journal of Physical Medicine & Rehabilitation. 2022 Jan;101(1):48-52.
29. Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re'em Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021 Aug 1;38:101019.
30. Oleson CV, Shermon S, Olsen AC. Spinal Cord Infarction and Ischemia Attributed to Severe Acute Respiratory Syndrome Coronavirus-2, with Progression to Post-Acute Sequelae of COVID-19: A case series. Presented at the Annual Meeting of the American Spinal Injury Association. St. Louis MO, USA July 8 2021.
31. Sneller MC, Liang CJ, Marques AR, Chung JY, Shanbhag SM, Fontana JR, et al. A longitudinal study of COVID-19 sequelae and immunity: baseline findings. Annals of Internal Medicine. 2022 Jul;175(7):969-79.
32. Lamb LE, Timar R, Wills M, Dhar S, Lucas SM, Komnenov D, et al. Long COVID and COVID-19-associated cystitis (CAC). International Urology and Nephrology. 2022 Jan;54:17-21.
33. Lamb LE, Dhar N, Timar R, Wills M, Dhar S, Chancellor MB. COVID-19 inflammation results in urine cytokine elevation and causes COVID-19 associated cystitis (CAC). Medical Hypotheses. 2020 Dec 1;145:110375.
34. Scivoletto G, Morganti B, Molinari M. Early versus delayed inpatient spinal cord injury rehabilitation: an Italian study. Archives of Physical Medicine and Rehabilitation. 2005 Mar 1;86(3):512-6.
35. Richard-Denis A, Dionne A, Bourassa-Moreau É, Khoueir PJ, Maurais G, Mac-Thiong JM. Does Wait Time During Acute Care for Transfer to Rehabilitation Admission Impact the Outcomes After a Traumatic Spinal Cord Injury?: A Retrospective Cohort Study. American Journal of Physical Medicine & Rehabilitation. 2022 Dec 1;101(12):1122-8.
36. Filipović T, Gajić I, Gimigliano F, Backović A, Hrković M, Nikolić D, et al. The role of acute rehabilitation in COVID-19 patients. European Journal of Physical and Rehabilitation Medicine. 2023 Jun;59(3):425-35.
37. Adjaye-Gbewonyo D, Vahratian A, Perrine CG, Bertolli J. Long COVID in Adults: United States, 2022. NCHS Data Brief no 480. Hyattsville, MD: National Center for Health Statistics. 2023.
38. Melamed E, Rydberg L, Ambrose AF, Bhavaraju-Sanka R, Fine JS, Fleming TK, et al. Multidisciplinary collaborative consensus guidance statement on the assessment and treatment of neurologic sequelae in patients with post-acute sequelae of SARS-CoV-2 infection (PASC). PM & R: The Journal of Injury, Function, and Rehabilitation. 2023 May;15(5):640-62.