Commentary
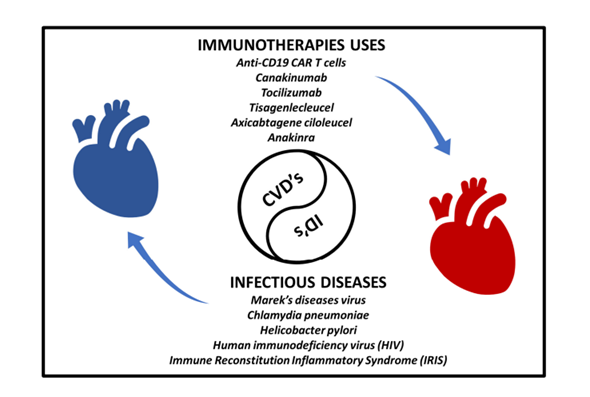
Cardiovascular disease, also known as heart disease, encompasses a class of medical conditions that affect the heart or blood vessels [1,2]. Cardiovascular disease is a leading cause of death worldwide, and it encompasses various disorders and conditions [3-5]. The recognition of the involvement of the heart in many diseases has been established for several decades. Cardiovascular disease is influenced by a wide range of microbial agents, which have a role in the development of cardiac-related disorders and the impact of disease etiology on heart functions. Infectious agents such as bacteria, viruses & fungi and their antigenic peptides impacted different cardiac components that involves pericardium, myocardium, endocardium, malfunctioning in valves, nervous system, and coronary arteries. Pericarditis (sac covering heart outer surface) and myocarditis (heart muscle) are recognized as long-haul of inflammation in specific layers of the heart, that can lead to heart failure or arrhythmia, additionally it can cause myocytes and neural conductive system. On the other hand, endocarditis defines as infection in lining of heart valves, that is called infective endocarditis or bacterial endocarditis (usually bacterial involvement). Infective endocarditis primarily affects cardiac valves and can be fatal if not treated. This commentary reviews the progress made in recent on understanding the immunopathology of infectious diseases and complication for the heart though all possible aspects, as well as potential novel approach for therapy. Various infectious diseases involved in cardiovascular complications has been represented in Figure 1.
Figure 1. Link between infectious diseases and cardiovascular disorders with possible immunotherapies implemented to mitigate the progression of cardiovascular diseases.
The hypothesis that infection has apathogenic role in cardiac diseases was first suggested by clinical observation by Fabricant et al. in late 70s [6]. Clinical observation showed that infection of chickens with Marek’s diseases virus, and avian herpesvirus, causes atherosclerotic lesions in coronary arteries and other vessels [7]. Similarly, Chlamydia pneumoniae and Helicobacter pylori show plaques formation in coronary arteries [7,8]. On the other hand, Influenza virus is associated with acute myocardial infarction [9] and human immunodeficiency virus (HIV) has been linked to atherosclerosis and other cardiovascular diseases through systemic inflammation [10]. Overall, infectious agents have long been recognized as causes of disease involving components of structure of heart- pericardium, muscle, endocardium, valves, autonomic nerves, and heart vessels. The spectra of microorganisms causing heart disease, broadly all class of microbes- i.e., viruses, bacteria, fungi, and parasites [11]. Mechanism causing heart diseases varies with pathogen specific, but all includes variety of cytokines and chemokines mediated inflammatory reactions that directly or indirectly damages heart walls and shows differential diseased pathology. The occurrence known as Immune Reconstitution Inflammatory Syndrome (IRIS) typically manifests in individuals with compromised immune systems, particularly those infected with HIV [12-15]. In the contemporary period, scientists are actively involved in the pursuit of developing immune suppression through immunotherapy within locally exacerbated microenvironments [16,17]. This approach stands in stark contrast to conventional cancer immunotherapy methods that have been observed thus far [18,19]. There is a substantial need for further contributions to our comprehension of the emerging field of cardio-immunology, which encompasses fundamental and translational mechanisms, as well as the clinical implications for managing patients [20]. Moreover, it is crucial to place a high priority on conducting extensive research in the realm of cardiovascular medicine and inflammatory infectious diseases, given the substantial difficulties and unaddressed requirements associated with these domains.
Immunotherapeutic Approaches
Within the complex network of inflamed myocardial tissue, numerous potential targets exist for the modulation of the immunological paradigm. The chimeric antigen receptor (CAR) construct comprises antigen recognition domains and intercellular signaling domains that have been genetically modified into cytotoxic T lymphocytes. The utilization of CAR-T cells targeting activated fibroblasts and senescent cells presents promising avenues for enhancing cardiac healing in cases of heart failure. Exacerbated inflammation induced by United States Food and Drug Administration (FDA)-approved anti-CD19 (Cluster of Differentiation 19) CAR T cells, specifically Tisagenlecleucel and Axicabtagene ciloleucel, can be effectively managed with the use of Interleukin-6 blockade, such as Tocilizumab [21,22]. The manipulation of macrophage polarization using graphene oxide to promote the M2-like population with reparative characteristics, along with the localized administration of Interleukin-4 at the site of inflammation, has the potential to yield advantageous outcomes in cases of myocardial infarction [23]. In a similar vein, modulating the ratio of eosinophils to neutrophils could potentially contribute to the process of recuperation. The study conducted by researchers has shown that the introduction of Eosinophil-specific Interleukin-4 and Mouse eosinophil-associated RNase (mEar1) expression effectively inhibits reactive oxygen species (ROS) and the subsequent death of cardiomyocytes [24]. The interruption of the B cell pathway through Programmed Cell Death Protein 1 (PD-1) mediation presents a potential advantage, as it leads to the elevation of cardiomyopathy-specific Immunoglobulin G (IgG) autoantibody levels in individuals with dilated cardiomyopathy [25]. The delivery of targeted interleukin blockade, such as Interleukin-1, Interleukin-11, Interleukin-15, Transforming growth factor-β (TGF-β), or the administration of reparative Interleukin-10, has the potential to mitigate the detrimental effects of excessive inflammation. Interleukin-1β is a multifunctional cytokine that exhibits diverse inflammatory properties in the context of acute infection, potentially leading to the onset or exacerbation of cardiac damage. The application of a systemic blocking strategy for interleukin-1 using Canakinumab, a competitive inhibitor, or Anakinra, an Interleukin-1β blocker, has demonstrated a limited yet favorable impact on animal models of myocardial infarction [26,27]. The examination of blocking similar Interleukin-11 and Interleukin-15 is now being conducted, as indicated by the Schafer et. al., and Guo et. al., [28,29]. Finally, the advancement of innovative chemical compounds and biological interventions, such as miRNA and exosomes, presents intriguing immunomodulatory possibilities [30].
Declarations
Authors declare no conflict of interest.
Availability of Data and Materials
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Funding
This work received no funding.
Authors' Contributions
CV, AT, VK: Main contributor in writing the manuscript; CV, AT, VK: Proofreading; CV, VK: Corresponding author.
Acknowledgements
Not applicable.
References
2. Rosenzweig R, Kumar V, Gupta S, Bermeo-Blanco O, Stratton MS, Gumina RJ, et al. Estrogen Receptor-β Agonists Modulate T-Lymphocyte Activation and Ameliorate Left Ventricular Remodeling During Chronic Heart Failure. Circ Heart Fail. 2022;15(7):e008997.
3. Kumar V, Rosenzweig R, Asalla S, Nehra S, Prabhu SD, Bansal SS. TNFR1 Contributes to Activation-Induced Cell Death of Pathological CD4. JACC Basic Transl Sci. 2022;7(10):1038-49.
4. Pawar VA, Srivastava S, Tyagi A, Tayal R, Shukla SK, Kumar V. Efficacy of Bioactive Compounds in the Regulation of Metabolism and Pathophysiology in Cardiovascular Diseases. Curr Cardiol Rep. 2023;25(9):1041-52.
5. Rosenzweig R, Gupta S, Kumar V, Gumina RJ, Bansal SS. Estrogenic bias in T-Lymphocyte biology: Implications for cardiovascular disease. Pharmacol Res. 2021;170:105606.
6. Fabricant CG, Fabricant J, Litrenta MM, Minick CR. Virus-induced atherosclerosis. J Exp Med. 1978;148(1):335-40.
7. Minick CR, Fabricant CG, Fabricant J, Litrenta MM. Atheroarteriosclerosis induced by infection with a herpesvirus. Am J Pathol. 1979;96(3):673-706.
8. Kowalski M. Helicobacter pylori (H. pylori) infection in coronary artery disease: influence of H. pylori eradication on coronary artery lumen after percutaneous transluminal coronary angioplasty. The detection of H. pylori specific DNA in human coronary atherosclerotic plaque. J Physiol Pharmacol. 2001;52(1 Suppl 1):3-31.
9. Warren-Gash C, Smeeth L, Hayward AC. Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: a systematic review. Lancet Infect Dis. 2009;9(10):601-10.
10. Triant VA. HIV infection and coronary heart disease: an intersection of epidemics. J Infect Dis. 2012;205 Suppl 3(Suppl 3):S355-61.
11. Huaman MA, Henson D, Ticona E, Sterling TR, Garvy BA. Tuberculosis and Cardiovascular Disease: Linking the Epidemics. Trop Dis Travel Med Vaccines. 2015;1.
12. Gouel-Cheron A, Nason M, Rupert A, Sheikh V, Robby G, Fahle GA, et al. Cardiovascular Biomarker Profile on Antiretroviral Therapy Is Not Influenced by History of an IRIS Event in People With HIV and Suppressed Viremia. Open Forum Infect Dis. 2020;7(1):ofaa017.
13. Kenyon C, Schrueder N, Ntsekhe M, Meintjes G. Heart failure and cardiogenic shock associated with the TB-immune reconstitution inflammatory syndrome. Cardiovasc J Afr. 2012;23(3):e14-7.
14. Verma C, Swami B, Upadhyay V, Singh A, Anang V, Saraswati S, et al. Tuberculosis and tuberculosis-associated immune reconstitution inflammatory syndrome in HIV: immunological review of manifestation and immunopathogenesis. HIV & AIDS Review International Journal of HIV-Related Problems. 2020;19(2):67-73.
15. Verma C, Sharma SK, Natarajan K, Sreenivas V, Upadhyay V, Sinha S, et al. Immunological alterations in tuberculosis-associated immune reconstitution inflammatory syndrome (TB-IRIS) in HIV-infected patients. The Indian Journal of Experimental Biology. 2019;57:786-95.
16. Engelen SE, Robinson AJB, Zurke YX, Monaco C. Therapeutic strategies targeting inflammation and immunity in atherosclerosis: how to proceed? Nat Rev Cardiol. 2022;19(8):522-42.
17. Pawar VA, Tyagi A, Verma C, Sharma KP, Ansari S, Mani I, et al. Unlocking therapeutic potential: integration of drug repurposing and immunotherapy for various disease targeting. Am J Transl Res. 2023;15(8):4984-5006.
18. Anang V, Singh A, Kottarath SK, Verma C. Receptors of immune cells mediates recognition for tumors. Prog Mol Biol Transl Sci. 2023;194:219-67.
19. Singh A, Anang V, Kumari K, Kottarath SK, Verma C. Role of lymphocytes, macrophages and immune receptors in suppression of tumor immunity. Prog Mol Biol Transl Sci. 2023;194:269-310.
20. Srivastava S, Pawar VA, Tyagi A, Sharma KP, Kumar V, Shukla SK. Immune Modulatory Effects of Ketogenic Diet in Different Disease Conditions. Immuno [Internet]. 2023; 3(1):[1-15 pp.].
21. Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):439-48.
22. Ruella M, June CH. Predicting Dangerous Rides in CAR T Cells: Bridging the Gap between Mice and Humans. Mol Ther. 2018;26(6):1401-3.
23. Han J, Kim YS, Lim MY, Kim HY, Kong S, Kang M, et al. Dual Roles of Graphene Oxide To Attenuate Inflammation and Elicit Timely Polarization of Macrophage Phenotypes for Cardiac Repair. ACS Nano. 2018;12(2):1959-77.
24. Liu J, Yang C, Liu T, Deng Z, Fang W, Zhang X, et al. Eosinophils improve cardiac function after myocardial infarction. Nat Commun. 2020;11(1):6396.
25. Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291(5502):319-22.
26. Abbate A, Salloum FN, Vecile E, Das A, Hoke NN, Straino S, et al. Anakinra, a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation. 2008;117(20):2670-83.
27. Toldo S, Mezzaroma E, Van Tassell BW, Farkas D, Marchetti C, Voelkel NF, et al. Interleukin-1β blockade improves cardiac remodelling after myocardial infarction without interrupting the inflammasome in the mouse. Exp Physiol. 2013;98(3):734-45.
28. Schafer S, Viswanathan S, Widjaja AA, Lim WW, Moreno-Moral A, DeLaughter DM, et al. IL-11 is a crucial determinant of cardiovascular fibrosis. Nature. 2017;552(7683):110-5.
29. Guo L, Liu MF, Huang JN, Li JM, Jiang J, Wang JA. Role of interleukin-15 in cardiovascular diseases. J Cell Mol Med. 2020;24(13):7094-101.
30. Piotto C, Julier Z, Martino MM. Immune Regulation of Tissue Repair and Regeneration via miRNAs-New Therapeutic Target. Front Bioeng Biotechnol. 2018;6:98.