Abstract
Background: Rheumatoid arthritis (RA) is a chronic, autoimmune disease that is associated with local and systemic inflammation, resulting in chronic pain and physical function limitations that may negatively impact quality of life (QOL). Despite advances in pharmacological therapies, currently available treatment options may be associated with adverse events and come at a high price tag. As a result, research efforts have grown to focus on nutritional interventions to support pharmacological therapies, reduce inflammation (targeting biomarkers of disease activity) and improve QOL.
Objectives: In this systematic review, data was collected on the most recent non-pharmacological interventions used in RA treatment. The efficacy and potential practical applications of various nutritional interventions used in the RA management will be discussed. This review has been divided into three parts. In this first section, we discuss five structured diets and their clinical impacts on patients with RA. The diets discussed in this article include the anti-inflammatory diet in RA (ADIRA) diet, elemental and elimination diets, weight loss, and a Mediterranean diet (MD). For more information on the other contents of this systematic review you may refer to Part 2: Supplementation and Part 3: Fruit and herbs.
Methods: A search of the literature was conducted to identify nutritional interventions in the progression and management of RA. Eligible study designs included meta-analyses, systematic reviews, randomized control trials (RCT) and prospective/retrospective studies. Exclusion criteria included non, in vivo human studies, n<40, cross-sectional studies, case-studies, and lack of access to available text.
Results: Initially, 334 articles were identified. After removing studies for lack of relevance, exclusion criteria, and duplicates, 22 articles remained. The eligible articles were divided into five groups based on design: meta analyses, systematic reviews, RCTs, literature reviews, and prospective studies. The eligible articles were grouped together based on intervention type: diets, supplementation and the implementation of fruits and herbs. Five articles were placed under the category of diet which includes one systematic review, two RCT and two literature reviews.
Conclusion: Dietary interventions may be an effective method for reducing inflammation and symptoms associated with RA. Significant improvements in indices of RA, such as the DAS-28 and HAQ, were observed with the use of a MD, vegan, and vegetarian diets as well as exercise and weight loss. These outcomes were supported by previous reviews demonstrating the safety and efficacy of these adjuvant therapies. This review also determined that the Anti-inflammatory Diet in RA (ADIRA) diet, as well as elimination and elemental diets all require further research before recommendations can be made on these interventions. However, past research has identified highly immunogenic foods which may induce symptoms and therefore clinicians should familiarize themselves with these triggers to educate their patients.
Keywords
Arthritis, Diet, Nutrition, Review, Rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is a chronic, autoimmune disease that is associated with local and systemic inflammation, resulting in chronic pain and physical function limitations that may negatively impact quality of life (QOL). This local inflammation (which is mediated by inflammatory cytokines and several host cells) manifests as joint swelling and joint cartilage erosion leading to pain, which can be severe and debilitating and result in functional limitations. Therefore, suppression of pain is an emphasis of treatment [1]. Pharmaceutical disease modifying agents have commonly been used for RA management. However, the high price tag and severe adverse event profile associated with these drugs may result in compliance issues and severe complications [2]. Research efforts have revealed that environmental and lifestyle factors, including diet, may play a role in RA presentation and progression, and modification of these factors may be associated with improvement in pain and QOL [3]. As such, the use of non-pharmaceutical interventions (such as dietary and nutritional modifications) to support pharmacological therapies and/or target underlying mechanisms of disease activity (reducing inflammation) may be of significant value to patients.
In particular, previous studies have indicated that a Mediterranean diet (MD), which includes increased intake of monounsaturated and polyunsaturated fatty acids, has been shown to improve disease status in RA [4]. Additional diets that have been investigated include plant-based diets (vegan and vegetarian), anti-inflammatory diets (ADIRA), elimination and elemental diets (which remove all but the most essential components of nutrition) as well as weight loss in general which can be achieved through a number of theses diets. This article provides a systematic review of currently available data on the use of non-pharmaceutical interventions for RA management, which was published between 2017 and 2020. The objective is to provide clinicians information on the safety and effectiveness of non-pharmaceutical interventions in RA management to aid in their recommendations. These recommendations include whether these non-pharmaceutical approaches may be used in combination with or alternatively to pharmaceutical agents.
In this effort, standardized assessments have been developed to ascertain both subjective and objective clinical improvements in disease activity. The Disease Activity Score in 28 joints (DAS-28) has been used to monitor disease progression and assesses tender joint counts (TJC), swollen joint counts (SJC), erythrocyte sedimentation rate (ESR), and a global health rating. The 36 item Short Form health survey (SF 36) is a subjective questionnaire utilized for gauging QOL. The visual analog scale (VAS) is an instrument used to measure subjective pain ratings by selecting a value from 0 (no pain) to 100 (severe pain). The health assessment questionnaire (HAQ) is a tool utilized for self-report of functional status. Additionally, the Ritchie score is a tool that is used to monitor disease severity. This score evaluates the tenderness of joint groups and scores them on a scale from zero to three. These indices will be used because they emphasize the role of both pain and function and can provide clinicians a means of understanding the impact of these treatments on disease status.
Our original search included 22 articles which were then subsequently divided into three sections, Dieting, Supplementation and Fruits and herbs. In this first of our 3-part series, we will discuss more formally structured dietary interventions and their clinical impacts on patients with RA. The diets discussed in this article include the anti-inflammatory diet in RA (ADIRA) diet, elemental and elimination diets, weight loss, and a MD. For more information on the other contents of this systematic review you may refer to Part 2: Supplementation and Part 3: Fruit and herbs.
Methods
Search strategy
A computer assisted systematic literature review was performed using PubMed for research articles examining nutritional interventions in the progression and management of RA. The PubMed word search included “Rheumatoid Arthritis Nutrition”, filtering for articles published between 2017 and 2020. The reference lists of retrieved articles were also considered when found to be relevant and if they fit the search criteria but were not discovered through individual searches. Relevance of these articles were assessed through a hierarchical approach that evaluated first the title, followed by the abstract, and the full manuscript. For articles that were not freely available, access was gained with Nova Southeastern University (NSU) library resources.
Selection criteria
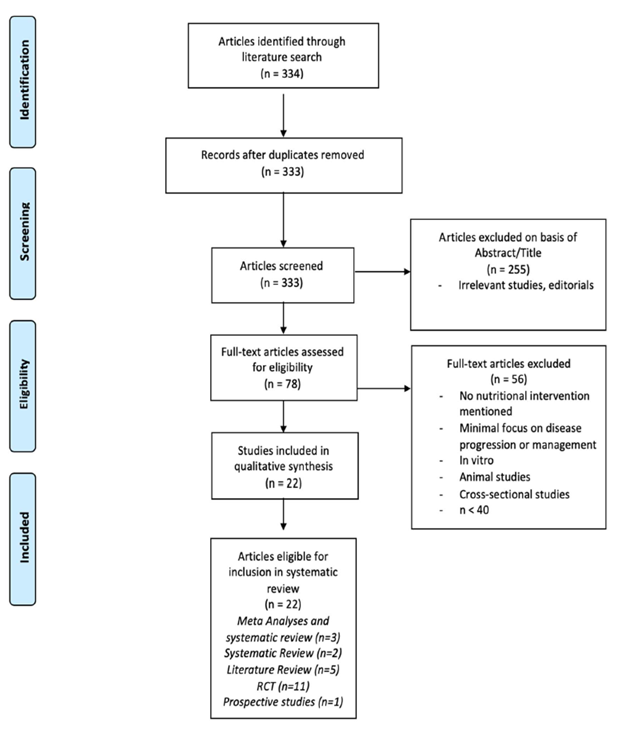
Studies were considered eligible if they discussed specific nutritional interventions evaluating the management or progression of active RA defined by criteria by the American College of Rheumatology (ACR) or European League Against Rheumatism (EULAR) [5]. Eligible study designs included meta-analyses, systematic reviews, randomized control trials (RCT), and prospective/retrospective studies. Exclusion criteria included non in vivo human studies, n<40, cross-sectional studies, case-studies, and lack of access to available text. A flow diagram (Figure 1) was developed using the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) 2009 outline [6].
Figure 1: Selection process flow diagram.
Study characteristics
Based on study design studies were divided into five groups:
Meta-analyses: 3
Systematic review: 2
RCT: 1
Literature reviews: 5
Prospective study: 1
Results
A total of 334 articles were identified from the initial electronic database search. Two hundred fifty-five articles were excluded based on lack of relevance found during title and abstract screening, and one duplicate was removed. Of the remaining 78 articles, 56 were excluded for the following reasons (based on exclusion criteria): Lack of in vivo human models, n<40, and lack of significant interventional study. A total of 22 articles remained for inclusion in this review.
|
Study |
Publication Year |
Subject |
Intervention(s) - duration |
Significant improvements |
|
Anti-inflammatory Diet in Rheumatoid Arthritis (ADIRA)-a Randomized, Controlled Crossover Trial Indicating Effects on Disease Activity - Vadell et al. [7] |
2020 |
50 |
ADIRA - Vadell et al. [7] – 10 weeks
|
None |
|
Managing Rheumatoid Arthritis with Dietary Interventions - Khanna et al. [9] |
2017 |
47 |
Elimination/Elemental diet- Kavanagh et al. [9] |
Compared to baseline: GS (p=0.008) Rtichie Score (p=0.006) |
|
2) 66 3) 53 4) 66 |
Vegetable/Vegan diet
|
1) VAS |
||
|
Nutrition Interventions in Rheumatoid Arthritis: The Potential Use of Plant-Based Diets. A Review - Alwarith et al. [27] |
2019 |
1) 66 2) 48 |
Elimination/Elemental diet
|
Compared to baseline: 1) 40.5% improvement in ACR20 |
|
1) 66 2) 53 3) 66 |
Vegetarian/Vegan diet
|
Compared to control: 2) At one month: CRP (p<0.0005) EMS (p<0.0002) ESR (p<0.002) GS (p<0.0005) HAQ (p<0.0001) pain score (p<0.0001) Ritchie's articular index (p<0.0004) SJC (p<0.04) TJC (p<0.0002)
3) DAS28 (p=0.047) CRP (p=0.008) |
||
|
1) 982 2) 174 3) 53 |
Weight loss 1) Schulman et al. [37] 2) Kreps et al. [38] 3) Sparks et al. [39] |
|
||
|
The effects of the Mediterranean diet on rheumatoid arthritis prevention and treatment: a systematic review of human prospective studies- Forsynth et al. [4] |
2017 |
1) 51 2) 130 |
Mediterranean diet
|
Compared to controls: 1) CRP (p=0.006), DAS-28 (p<0.001), HAQ (p<0.05), physical functioning (p=0.006). SJC and VAS pain
2) Follow up visits 3 months: pain (p=0.011) and physical functioning (p=0.03) 6 months: EMS (p=0.041), HAQ (p<0.05) and VAS (p=0.002) |
|
Effect of a Dynamic Exercise Program in Combination with Mediterranean Diet on Quality of Life in Women with Rheumatoid Arthritis - Garcia-Morales et al. [20] |
2019 |
144 |
Mediterranean Diet + exercise- Garcia-Morales et al. [20] - 24 weeks |
Compared to controls: SF-36 |
Discussion
ADIRA diet
ADIRA-A randomized, controlled crossover trial indicating effects on disease activity
In a study conducted on a Swedish population, Vadell et al. performed a 10-week, single-blinded, crossover study, where 50 patients with RA were randomly assigned to a diet consisting of anti-inflammatory foods or a control diet comparable to the general dietary intake in Sweden [7]. The interventional diet emphasized increased proportional intake of fruit, fiber, fish, and probiotics. The diet was referred to as the “fiber” diet whereas the control was referred to as the “protein” diet to mask the intervention. Fiber as well as n-3 PUFA (polyunsaturated fatty acid) content was higher in the intervention at 24 g/d (5.2 g/MJ) and 4 g/d (3 Energy%) respectively as compared with the 8.3 g/d (1.8 g/MJ) and 0.8 g/d (0.6 E%) in the control diet. One concern regarding this study is the difficulty in blinding a diet where individuals involved are likely to have preconceived notions about which foods are inherently healthier. Furthermore, this diet was performed on a local Swedish population and therefore the results may not be as generalizable to other populations subject to different diets and genetics. Originally improvements were discovered in the interventional group as compared to the control, however, upon ANCOVA analysis, the study indicated no significant difference in DAS28-ESR score between the groups.
Section summary
Overall, due to failure to reach statistical significance on intervention after ANCOVA analysis and the lack of generalization beyond the Swedish population, we do not feel this study supports the recommendation of its proposed diet.
Elimination and elemental diets
Managing rheumatoid arthritis with dietary interventions
Elemental diets contain hypoallergenic foods with essential nutrients for daily requirements and are less immunogenic as compared to the average diet [8]. Kavanagh et al. [9] reported the effects of elemental diets on clinical and symptomatic parameters in patients with food-aggravated disease conditions. Forty-seven RA patients were provided an elemental diet or their usual diet (control). The elemental diet was given to induce remission and then was gradually adapted to reintroduce foods. When reintroduced foods were seen to induce symptoms, they were promptly removed from the diet. Statistically significant improvements were seen in Grip Strength (GS) (p=0.008) and Ritchie Score (p=0.006) but were not seen with ESR, CRP, thermographic joint score, or functional score. Any improvements that were observed with the treatment of an elemental diet quickly diminished upon discontinuation [9]. Limitations of this study included the inability to blind subjects leading to emphasis on subjective measurements of improvement in addition to high dropout rates with only 38 percent of patients completing the study.
Nutrition interventions in rheumatoid arthritis: The potential use of plant-based diets. A review.
A 2019 review on the potential use of plant-based diets by Alwarith et al. [10] discussed elimination and elemental diets research efforts in RA management. As new research efforts indicate possible gastrointestinal links to the causes of RA [3], attention has begun to be placed on food sensitivities and the involvement of certain antigens as seen in other autoimmune diseases [11]. Elimination diets remove specific components from one’s diet in order to determine if it plays a role in the symptoms of interest, whereas an elemental diet involves specific selection of foods involving eliminating all antigenic proteins from one’s diet. Referenced in the review was a 2001 clinical trial by Hafström [12] with 66 RA patients on a vegan diet absent of gluten for a nine-month period. Results of the study indicated a 40.5 percent improvement in the ACR20 improvement criteria compared with four percent in the non-vegan group [12]. Meanwhile, Alwarith’s review indicated that responses to these methods can be highly variable and any improvement may quickly disappear upon resumption of previous diets (as seen in the study). Darlington et al. reported that about 75 percent of those that responded to an elimination diet resulted in improvements in subjective and objective measurements of disease activity [13]. Still, identifying individual food sensitivities has also been described as a challenge with these methods of treatment. Often skin prick testing is used to identify Immunoglobulin (Ig)E mediated responses to a stimulus, although this has not always correlated with offensive foods [14]. Another study was performed by Darlington et al. [15] using elimination and oral food challenges in 48 RA patients to identify foods capable of inducing symptoms in this population. Participants underwent a six-week elimination diet, which identified 20 different foods. Of these foods, corn and wheat were the most common to incite symptoms, seen in 57 percent and 54 percent of participants, respectively. These findings are summarized in Table 2 below. Furthermore, a 2009 systematic review by Hagen et al. [16] assessed the efficacy and safety of dietary interventions in the treatment of RA. Two clinical trials evaluating the effects of vegan and elimination diets were included. Both trials evaluated the effectiveness in RA symptom improvement using a four-week elemental diet as compared to controls but due to inadequate reporting resulted in inconclusive findings [16].
Section summary
Overall, extremely little data has been reported on non-pharmaceutical interventions and further research is required to determine effectiveness of these dietary modifications in identifying RA triggers and improving symptomatology. Many of these studies resulted in high dropout rates and regression of disease severity upon cessation of treatment, which brings in to question the efficacy of their application in a clinical setting. Meanwhile, regarding the foods commonly known to cause immunogenic responses, clinicians should encourage patients to be conscious of the components in their diet in order to minimize exacerbations and manage symptoms.
|
Food |
% patients affected |
Food |
% patients affected |
|
Corn |
57 |
Malt |
27 |
|
Wheat |
54 |
Cheese |
24 |
|
Bacon/pork |
39 |
Grapefruit |
24 |
|
Oranges |
39 |
Tomato |
22 |
|
Milk |
37 |
Peanuts |
20 |
|
Oats |
37 |
Sugar (cane) |
20 |
|
Rye |
34 |
Butter |
17 |
|
Eggs |
32 |
Lamb |
17 |
|
Beef |
32 |
Lemon |
17 |
|
Coffee |
32 |
Soy |
17 |
Mediterranean diet and exercise
The effects of the Mediterranean diet on RA prevention and treatment: A systematic review of human prospective studies
Of the various interventions discussed, the MD has garnered much attention in recent years. A 2017 systematic review by Forsynth et al. [4] evaluated the effects of MD on prevention and management of RA. For this review, we will focus on the treatment aspect of the Forsynth article. The MD is characterized by the consumption of olive oil, unrefined cereals, fresh or dried fruit and vegetables, moderate amounts of fish, dairy, and meat in addition to spices and wine [17]. The reason this diet has garnered so much interest is because of its potential anti-inflammatory effects [18], which for the reasons previously discussed may benefit patients suffering with RA. In 2003, an RCT was conducted by Sköldstam et al. [19], which evaluated the effect of a Cretan MD (modified to include more dairy products) versus the typical Western diet on the suppression of disease activity in patients with newly diagnosed RA over a period of three months. The initial three-week phase of the study was conducted in a hospital setting, in which participants were provided meals resembling the desired diet as well as six lessons about Mediterranean food and cooking in attempt to maintain compliance in the following nine weeks outside of the hospital setting. The control group received no recommendations. Compliance was determined through questionnaires, interviews, and measurement of biological markers. When compared to the control group the MD group demonstrated improvements in disease activity (p<0.001), physical functioning (p=0.006), and inflammatory biomarkers (i.e., CRP) (p=0.006). However, no significant changes were observed in terms of morning stiffness or changes in medication dose. At week 12, the intervention group reported significant reductions in the DAS28 score (p<0.001), while no change was seen in the control group.
The other study included in the review was a clinical control trial (CCT) conducted by McKellar et al. [20]. This study included 130 female patients diagnosed with RA who were assigned to an intervention group (n=75) or a control group (n=55). The intervention group attended a six-week cooking course in addition to being provided information about the MD and relevant cooking practices, whereas the control group was provided with materials on general principles of a healthy diet. Compliance to the diet was determined through food frequency questionnaires and laboratory markers measured at the initial and follow-up visits. The findings showed significant improvements in parameters measured at the three month follow-up including pain (p=0.011) and physical functioning (p=0.03) as well as at the six-month follow-up in the global visual analogue scale (p=0.002) and morning stiffness (p=0.041) as compared to the control group. However, no change was reported in the DAS28 score for both groups. Additionally, both interventions by Sköldstam and McKellar indicated a decrease in HAQ score for the MD group at six-month follow-up (p<0.05).
Section summary
Limitations of the studies performed by Sköldstam and McKellar included that random allocation was not feasible and both groups solely included women. Further criticism of McKellar’s study includes that statistical analysis was performed on two groups at different points in time in addition to most measures of disease activity being subjective. Lastly, the large amount of time spent with the intervention group could have additionally resulted in a placebo effect thus overall leading to this study having a high risk of bias.
The studies discussed by Sköldstram [19] and McKellar [20] are also included in works by Alwarith et al. [10] and Petersson [21], both resulting in similar conclusions as this review and therefore will not be further elaborated on in this section. Forsynth went on to acknowledge that this systematic review was limited due to the inclusion of only a small number of interventions that met their criteria.
Effect of a dynamic exercise program in combination with MD on QOL in women with rheumatoid arthritis
Garcia-Morales et al. performed an RCT that included 144 participants with RA to compare the effectiveness of a MD and exercise. The study was divided into four groups: 1) MD, 2) dynamic exercise program (DEP), 3) MD+DEP and 4) controls [22]. These groups contained 40, 37, 36 and 31 participants, respectively. The MD was developed by a dietician and prescribed according to basal energy expenditure estimated using the Harris-Benedict equation [23]. It consisted of 50 percent carbohydrates, 30 percent fats, and 20 percent proteins. The diet was based on the consumption of olive oil or canola oil as the main dietary fat, whole grains (one to two portions/meal), fruits (two to four portions/day), vegetables (two to three portions/meal), fish (greater than two portions/week), oilseeds (one to two portions/day), legumes (greater than two portions/week), and red meat (greater than two portions/week). In addition, all participants received individual verbal instructions from the dietician as well as a nutritional handbook containing menus for example meals. The DEP consisted of an 80 to 90-minute training sessions twice per week, which was a five-stage combination of warm-up, aerobic exercise, anaerobic exercise, recreational sports, and cool down. Exercise adherence was determined if patients attended 80 percent of exercise sessions. Both the DEP and the control group received general nutrition recommendations.
Results of the study indicated there were statistically significant changes on the SF-36 global health, global punctuation, and mental health components compared to control for DEP, MD, and MD+DEP groups. The improvements in all components were greatest for the MD+DEP group, with the exception of the mental component, which the greatest change was in the DEP alone. A potential explanation for this may be that adhering to a new diet could serve as an added mental stressor. Notably, this study also showed statistically significant changes on the SF-36 physical component within the DEP and MD+DEP groups, but not for MD alone. Limitations of this study included that it only involved a female population as well as that it only included patients with low (<3.2) DAS-28 scores and therefore, may be less generalizable.
Previous studies have evaluated the benefits of various exercise plans in RA patients including Bilberg [24], Badsha [25], and Brorsson [26]. These studies implemented aquatic exercise programs, yoga, and hand exercises, respectively. Bilberg reported significant improvements in vitality as determined by the SF-36 and improvements in muscular function (p<0.005) as compared to controls. These improvements were seen to last about three months. Badsha indicated that patients in an eight-week yoga program resulted in statistically significant improvements in DAS-28 as well as HAQ but not QOL. Lastly, Brorsson demonstrated improved hand function (p<0.01) and force (p<0.001) after six weeks of exercise.
Based on this data, it appears that a MD in addition to a twice weekly exercise regimen may result in improvements in the QOL in women with RA with low DAS-28 scores. Furthermore, Garcia-Morales emphasizes the importance of the duration of the intervention in order to obtain and maintain significant improvements as was seen in Bilberg’s study. Furthermore, Garcia-Morales states that a period of at least 24 weeks should be used to observe the benefits of this dual intervention. Overall, this study indicated that combinations in both diet and exercise result in greater improvements than either one alone on the disease state of female RA patients.
Section summary
The information regarding the effectiveness of MD among RA patients is limited, with the data included in this review resulting from two trials that have been circulated throughout various publications [4,10,21]. In addition, the findings from these studies indicated high levels of bias and the results must be approached with caution. Meanwhile, the results of these two studies in addition to the study performed by Garcia-Morales, indicate that the MD demonstrates the ability to improve the disease state of patients with RA. With the largest concern being compliance, this diet is safe and practical for clinicians to recommend to their patients. The impact of this diet can be maximized through the incorporation of concomitant exercise. At this time, we recommend the MD in addition to exercise for RA patients, with the caveat that further studies should be performed in order to strengthen these recommendations and provide additional data.
Vegetarian/vegan diets
Managing rheumatoid arthritis with dietary interventions
The article by Khanna discusses a 2001 meta-analysis conducted by Müller et al. in order to determine the effects of fasting followed by a vegetarian diet in RA patients [8,27]. Müller’s search yielded 31 original articles that were critically analyzed. The main focus of the review, however, was on four controlled studies [28,29], which included the work of Kjeldsen-Kragh et al. [28] that is discussed in detail in the following article of this section. Two of these four controlled trials were randomized. Pooling of the p-values from these studies when combined illustrated significant results (p<0.0001). Müller’s review indicated that the literature analyzed supports the hypothesis that short periods of fasting (seven to 23 days) followed by a vegetarian diet (3.5 months or more) can cause statistically significant and clinically relevant long-term improvements in RA patients. These improvements were primarily assessed using VAS. They did, however, acknowledge limitations to the development of any final conclusions due to the small number of methodologically sound studies, potential publication bias that may have limited their exposure to other articles that may have otherwise fit into the analysis and that their findings cannot be generalized to the general population because a majority of patients were eager to fast and were compliant throughout these rigorous procedures. Lastly, the composition of these fasts was poorly defined in the analysis thus making it more difficult to compare results.
Other works discussed in this Khanna’s review include Hafström [12] and Elkan et al. [30], which are discussed in more detail in ‘Nutrition interventions in rheumatoid arthritis: The potential use of plant-based diets. A review’ by Alwarith et al. [10]
Nutrition interventions in RA: The potential use of plant-based diets. A review.
Many studies have linked inflammatory markers, such as c-reactive protein (CRP), IL-6, and homocysteine, with the consumption of red meat and animal-based products [31], while others have demonstrated the potential of vegan and vegetable-based diets to reduce these inflammatory markers [32,33]. With the knowledge that RA is a disease mediated by these same inflammatory markers, it is reasonable to assume the implementation of such a diet would result in clinical improvements. In the same clinical trial by Hafström [12] mentioned above, it was discovered that a gluten-free, vegan diet in RA patients resulted in the reduction of IgG, which is an antibody with pro-inflammatory properties and is often found to be elevated in this population. Additionally, Alwarith’s review evaluated a 1991 randomized, single-blind controlled trial with RA patients by Kjeldsen-Kragh [10,28]. This study evaluated the effects of a seven to 10 day fast, followed by 3.5 months of a gluten-free, vegan diet with a gradual shift to a vegetarian diet that occurred over the course of one year. Both the intervention and the control group stayed at a convalescent home during the first four weeks of the study. After one month, the diet group began to display improvements in the number of tender and swollen joints, Ritchie's articular index, pain score, duration of morning stiffness, grip strength (GS), erythrocyte sedimentation rate (ESR), CRP, white blood cell (WBC) count, and HAQ score which were maintained after one year [28]. In comparison, after one month, the control group displayed significant improvement in pain score alone. Another study referenced in Alwarith’s review by Elkan et al briefly discussed 66 RA patients that were randomly assigned to either a gluten-free, vegan diet or a well-balanced non-vegan diet for one year [30]. Disease activity was measured with the DAS-28 index as well as the European League Against Rheumatism (EULAR) response criteria at three and 12 months. DAS-28 was found to be higher in the control group (p=0.047), whereas the HAQ score was not significantly different. In addition, the vegan group was shown to have weight and body mass index (BMI) reductions by 4.2 kg and 1.4, respectively. In addition, CRP levels decreased from 13 to five at 12 months (p=0.008) [30]. No serious adverse effects were reported from these studies. However, in the study by Elkan, participants were supplemented with vitamin B12 to avoid any deficiencies.
Section summary
The studies discussed in this section were limited and combined a mixture of fasting, vegetarian, and vegan diets. Because of these reasons it is difficult to separate each aspect in order to determine how the application of either one could benefit patients with RA. However, the data available does indicate that vegan and vegetarian diets are capable of providing significant improvements in clinical parameters such as the DAS-28 and VAS. Although Kjeldsen-Kragh’s study demonstrated several other significant improvements when compared to Elkan’s study, it is difficult to determine if this was due to the addition of a fasting period. Currently, we believe there is sufficient data to recommend the incorporating of either a vegan or vegetarian diet to improve clinical symptoms. Still, future research should aim to determine the role of fasting as well as compare the benefits of a vegan versus vegetarian diet for more a concrete understanding of effectiveness.
Weight loss
Nutrition interventions in RA: The potential use of plant-based diets. A review.
While adipose tissue previously believed to be inert, it is well understood that adipose tissue is an organ of its own that can result in the production of pro-inflammatory cytokines that may contribute to the inflammatory processes seen in many disease states [34]. When considering that the inflammatory process in RA results in diffuse joint erosion, it is important to consider that adding additional weight may further exacerbate this damage. Several studies have indicated that RA patients that are overweight have worse outcomes than those with a normal BMI (≤24.9) [35,36] and therefore, a healthy weight should be addressed and maintained. The Canadian Early Arthritis Cohort (CATCH) study, which included 982 participants, indicated that being overweight or obese was independently associated with a decreased chance for achieving and sustaining remission. These patients were 25 percent and 47 percent less likely to achieve remission within three years, respectively [37]. In addition, a retrospective analysis performed in 2018 by Kreps et al., which included 174 participants with RA found that overweight individuals who lost ≥5 kg had a three times increased likelihood of improvement in disease activity compared to those who lost <5 kg. This study utilized the DAS28 and concluded that each kg of weight lost was associated with an improvement in the clinical disease activity index by 1.15 (p=.0026) [38]. Similar findings were seen by Sparks et al. when investigating weight loss in 53 RA patients post-bariatric surgery. Twelve months after surgery, six percent of patients were considered to have moderate to high disease activity as compared to 57 percent beforehand. A follow up 5.8 years after surgery indicated that 74 percent of patients were considered to be in remission when compared to 26 percent before the intervention. Additional data was collected from inflammatory markers, which were measured at six months, 12 months, and at 5.8 years and were found to be significantly lower (p<0.05, p<0.001, and p<0.001, respectively) [39].
Section summary
With evidence indicating the correlation between healthy BMI, weight loss, and the likelihood of both achieving and sustaining remission it is imperative that clinicians emphasize this aspect of management with their patients. With worsening disease activity comes increasing difficulty with daily activities and thus nutritional goals outlining desired weight and BMI should be regularly included as part of clinical practice in addition to pharmacologic therapy.
Conclusion
The goal of this review was to evaluate recent dietary interventions for clinical improvements and summarize recommendations that may serve as a tool for clinicians to confidently provide updated information to their patients. Of the various complimentary methods that can be implemented in RA patients, various diets have been suggested to improve symptoms and overall well-being. Most diets focus on eliminating “inflammatory” foods and adopting an eating pattern that reduces inflammation. There is insufficient evidence to support the efficacy of an ADRIA and elimination diet. However, improvements were observed in MD and plant-based diets. There is a strong relationship between healthy weight and severity of symptoms. The evidence suggests that maintaining a healthy weight along with regular physical activity correlates with lower disease activity. When used in conjunction with an individual’s current treatment, evidence supports dietary patterns, which encourage consumption of vegetables, n-3 PUFA, and minimally processed foods. These patterns are consistent with the MD. In addition to diet, clinicians should encourage patients to participate in regular exercise and maintain a healthy weight to further improve clinical parameters in patients with RA.
Acknowledgements
AM and KB performed the systematic review. MK validated the systematic search. AM, KB and CE wrote the paper. AM conceptualized the manuscript and MK reviewed the manuscript. Authors would like to extend their gratitude to Beth Gilbert, Instructor, Science Grant Writer, Kiran C. Patel College of Osteopathic Medicine, Nova Southeastern University for editing their manuscript.
Funding
There was no funding received for this study.
Compliance with Ethical Standards
Conflict of interest
All the authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
2. Benjamin O, Bansal P, Goyal A, Lappin SL. Disease modifying anti-rheumatic drugs (DMARD). StatPearls [Internet]. 2020 Feb 27.
3. Toivanen P. Normal intestinal microbiota in the aetiopathogenesis of rheumatoid arthritis. Annals of the Rheumatic Diseases. 2003 Sep 1;62(9):807-11.
4. Forsyth C, Kouvari M, D’Cunha NM, Georgousopoulou EN, Panagiotakos DB, Mellor DD, et al. The effects of the Mediterranean diet on rheumatoid arthritis prevention and treatment: a systematic review of human prospective studies. Rheumatology International. 2018 May 1;38(5):737-47.
5. Kay J, Upchurch KS. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology. 2012 Dec 1;51(suppl_6):vi5-9.
6. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Medicine 6(7): e1000097
7. Vadell AK, Bärebring L, Hulander E, Gjertsson I, Lindqvist HM, Winkvist A. Anti-inflammatory Diet In Rheumatoid Arthritis (ADIRA)—a randomized, controlled crossover trial indicating effects on disease activity. The American Journal of Clinical Nutrition. 2020 Jun 1;111(6):1203-13.
8. Khanna S, Jaiswal KS, Gupta B. Managing rheumatoid arthritis with dietary interventions. Frontiers in Nutrition. 2017 Nov 8;4:52.
9. Kavanagh R, Workman E, Nash P, Smith M, Hazleman BL, Hunter JO. The effects of elemental diet and subsequent food reintroduction on rheumatoid arthritis. Rheumatology. 1995 Mar 1;34(3):270-3.
10. Alwarith J, Kahleova H, Rembert E, Yonas W, Dort S, Calcagno M, et al. Nutrition interventions in rheumatoid arthritis: the potential use of plant-based diets. A review. Frontiers in Nutrition. 2019 Sep 10; 6:141.
11. van de Laar MA, Van der Korst JK. Rheumatoid arthritis, food, and allergy. In: Seminars in Arthritis and Rheumatism 1991; 21:12-23.
12. Hafström I, Ringertz B, Spångberg A, Von Zweigbergk L, Brannemark S, Nylander I, et al. A vegan diet free of gluten improves the signs and symptoms of rheumatoid arthritis: the effects on arthritis correlate with a reduction in antibodies to food antigens. Rheumatology. 2001 Oct 1;40(10):1175-9.
13. Darlington LG, Ramsey NW, Mansfield JR. Placebo-controlled, blind study of dietary manipulation therapy in rheumatoid arthritis. The Lancet. 1986 Feb 1;327(8475):236-8.
14. Gamlin L, Brostoff J. Food sensitivity and rheumatoid arthritis. Environmental Toxicology and Pharmacology. 1997 Nov 1;4(1-2):43-9.
15. Darlington LG, Ramsey NW. Review of dietary therapy for rheumatoid arthritis. Rheumatology. 1993 Jun 17;32(6):507-14.
16. Hagen KB, Byfuglien MG, Falzon L, Olsen SU, Smedslund G. Dietary interventions for rheumatoid arthritis. Cochrane Database of Systematic Reviews. 2009; 2009:CD006400.
17. UNESCO (2010) UNESCO: The Mediterranean Diet. United Nations Educational, Scientific and Cultural organization. Accessed 18 May 2017
18. Panagiotakos DB, Georgousopoulou EN, Pitsavos C, Chrysohoou C, Skoumas I, Pitaraki E, et al., ATTICA Study Group. Exploring the path of Mediterranean diet on 10-year incidence of cardiovascular disease: the ATTICA study (2002–2012). Nutrition, Metabolism and Cardiovascular Diseases. 2015 Mar 1;25(3):327-35.
19. Sköldstam L, Hagfors L, Johansson G. An experimental study of a Mediterranean diet intervention for patients with rheumatoid arthritis. Annals of the rheumatic diseases. 2003 Mar 1;62(3):208-14.
20. McKellar G, Morrison E, McEntegart A, Hampson R, Tierney A, Mackle G, et al. A pilot study of a Mediterranean-type diet intervention in female patients with rheumatoid arthritis living in areas of social deprivation in Glasgow. Annals of the Rheumatic Diseases. 2007 Sep 1;66(9):1239-43.
21. Petersson S, Philippou E, Rodomar C, Nikiphorou E. The Mediterranean diet, fish oil supplements and Rheumatoid arthritis outcomes: Evidence from clinical trials. Autoimmunity Reviews. 2018 Nov 1;17(11):1105-14.
22. García-Morales JM, Lozada-Mellado M, Hinojosa-Azaola A, Llorente L, Ogata-Medel M, Pineda-Juárez JA, et al. Effect of a Dynamic Exercise Program in Combination With Mediterranean Diet on Quality of Life in Women With Rheumatoid Arthritis. Journal of Clinical Rheumatology: Practical Reports on Rheumatic & Musculoskeletal Diseases. 2019 May 28.
23. Harris JA, Benedict FG. A biometric study of human basal metabolism. Proceedings of the National Academy of Sciences of the United States of America. 1918 Dec;4(12):370.
24. Bilberg A, Ahlmen M, Mannerkorpi K. Moderately intensive exercise in a temperate pool for patients with rheumatoid arthritis: a randomized controlled study. Rheumatology. 2005 Apr 1;44(4):502-8.
25. Badsha H, Chhabra V, Leibman C, Mofti A, Kong KO. The benefits of yoga for rheumatoid arthritis: results of a preliminary, structured 8-week program. Rheumatology International. 2009 Oct 1;29(12):1417-21.
26. Badsha H, Chhabra V, Leibman C, Mofti A, Kong KO. The benefits of yoga for rheumatoid arthritis: results of a preliminary, structured 8-week program. Rheumatology International. 2009 Oct 1;29(12):1417-21.
27. Müller H, De Toledo FW, Resch KL. Fasting followed by vegetarian diet in patients with rheumatoid arthritis: a systematic review. Scandinavian Journal of Rheumatology. 2001 Jan 1;30(1):1-0.
28. Kjeldsen-Kragh J, Borchgrevink CF, Laerum E, Haugen M, Eek M, Førre O, Mowinkel P, et al. Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis. The Lancet. 1991 Oct 12;338(8772):899-902.
29. Sköldstam L. Preliminary reports: fasting and vegan diet in rheumatoid arthritis. Scandinavian journal of rheumatology. 1986 Jan 1;15(2):219-21.
30. Elkan AC, Sjöberg B, Kolsrud B, Ringertz B, Hafström I, Frostegård J. Gluten-free vegan diet induces decreased LDL and oxidized LDL levels and raised atheroprotective natural antibodies against phosphorylcholine in patients with rheumatoid arthritis: a randomized study. Arthritis Research & Therapy. 2008 Apr 1;10(2):R34.
31. Nettleton JA, Steffen LM, Mayer-Davis EJ, Jenny NS, Jiang R, Herrington DM, et al. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA). The American Journal of Clinical Nutrition. 2006 Jun 1;83(6):1369-79.
32. Paalani M, Lee JW, Haddad E, Tonstad S. Determinants of inflammatory markers in a bi-ethnic population. Ethnicity & Disease. 2011;21(2):142.
33. Sutliffe JT, Wilson LD, de Heer HD, Foster RL, Carnot MJ. C-reactive protein response to a vegan lifestyle intervention. Complementary therapies in medicine. 2015 Feb 1;23(1):32-7.
34. Lu B, Hiraki LT, Sparks JA, Malspeis S, Chen CY, Awosogba JA, et al. Being overweight or obese and risk of developing rheumatoid arthritis among women: a prospective cohort study. Annals of the Rheumatic Diseases. 2014 Nov 1;73(11):1914-22.
35. Gremese E, Carletto A, Padovan M, Atzeni F, Raffeiner B, Giardina AR, et al. Obesity and reduction of the response rate to anti–tumor necrosis factor α in rheumatoid arthritis: an approach to a personalized medicine. Arthritis Care & Research. 2013 Jan;65(1):94-100.
36. Sandberg ME, Bengtsson C, Källberg H, Wesley A, Klareskog L, Alfredsson L, et al. Overweight decreases the chance of achieving good response and low disease activity in early rheumatoid arthritis. Annals of the Rheumatic Diseases. 2014 Nov 1;73(11):2029-33.
37. Schulman E, Bartlett SJ, Schieir O, Andersen KM, Boire G, Pope JE, et al. Overweight, obesity, and the likelihood of achieving sustained remission in early rheumatoid arthritis: results from a multicenter prospective cohort study. Arthritis Care & Research. 2018 Aug;70(8):1185-91.
38. Kreps DJ, Halperin F, Desai SP, Zhang ZZ, Losina E, Olson AT, et al. Association of weight loss with improved disease activity in patients with rheumatoid arthritis: A retrospective analysis using electronic medical record data. International Journal of Clinical Rheumatology. 2018;13(1):1.
39. Sparks JA, Halperin F, Karlson JC, Karlson EW, Bermas BL. Impact of bariatric surgery on patients with rheumatoid arthritis. Arthritis Care & Research. 2015 Dec;67(12):1619-26