Abstract
Multiple species obtain repetitive head collisions throughout the course of their lifetimes with minimal neurologic deficit. Nature has allowed the unique development of multiple protective mechanisms to help prevent neurotrauma. In this review, we examine the concept of rapid brain movement within the skull ‘Slosh’ and what nature teaches on how to prevent this from occurring. We look at individual animals and the protective mechanisms at play. Marching from macroscopic down to the molecular level, we pinpoint key elements of neuroprotection that are likely contributing. We also introduce new concepts for neuroprotection and address avenues of further discovery.
Keywords
Neurotrauma, Slosh, Nature, Neuroprotection, Emerging strategies
Introduction
Lessons from Nature
In nature, it is common for certain animals to experience repetitive head impacts with minimal to no head damage and/or long-term consequences. Specifically, birds such as the woodpecker and the diving gannet, along with horned/antlered animals like the bighorn sheep and deer engage in activities that necessitate head impacts on a routine basis [1]. Woodpeckers strike trees to look for food and develop nests. Diving gannets strike the water at high speed to catch prey. Horned animals compete for mates by head butting.
On average, woodpeckers slam their beak into tree trunks at a rate of 18-22 times per second, to either search for food or to create a nest [2]. This translates to woodpeckers moving their heads at 15 miles per hour. With each peck, a woodpecker undergoes a force between 1,200-1,400 times that of Earth’s gravity (g) [3]. To put this into perspective, a sudden implementation of 50 g would detach the connective tissue that surrounds much of a human’s organs [3]. Another bird that frequently experiences head impacts are the diving gannets. Diving gannets forage for food in the ocean. Diving gannets make 20-100 dives per foraging trip, and with each dive, the head of a diving gannet can collide with the surface of the water at speeds up to 20 meters per second [4]. Furthermore, animals such as the big-horned sheep use their horns as weapons in combat to compete for mates or guard territory. The cranial impact of a collision of horns between two big-horned sheep can be up to 3400 newtons per collision [5].
Unique anatomical characteristics of these animals enable the repetitive head collisions with limited deleterious consequences. Specifically, the woodpecker has a small subdural space and a unique tongue structure that limits brain movement [6]. The tongue of a woodpecker is notable because it extends posteriorly from the base of the mouth, around the neck, over the occiput, and into the right nostril. This creates a muscular sling inside the head of woodpeckers which has previously been identified as a possible shock absorber. In addition, the hyoid bone has been shown to aid the dissipation of energy during impact. Similarly, the horn of a big-horned sheep is a large, hollow curled structure primarily composed of keratin [7]. The distinctive tapered spiral horn geometry reduces the load more efficiently than other geometries, such as a tapered bar [7].
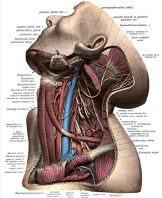
In contrast, although the human anatomy clearly differs from that of a woodpecker or big-horned sheep, the tightening of the muscles around the internal jugular vein (IJV) may possibly reduce the trauma experienced by an individual during head collision (Figure 1). The proposed principle is that by increasing venous engorgement, the ability for the brain to move within the cerebrospinal fluid becomes limited. This phenomenon of constricting neck muscles for impact is commonly observed amongst boxers and may indicate why they are able to sustain repeated forces of great magnitude. The development of collars that can aid athletes and warfighters is also a topic of ongoing investigation. The remainder of this review will look at the principles that mediate brain protection in association with traumatic brain injury (TBI).
Figure 1: Anatomical relationship of omohyoid and internal jugular vein.
Mechanisms of Energy Transfer: Shortcomings of Helmets
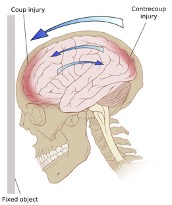
The injury process in traumatic brain injury often involves coup-contrecoup injury. The coup and contrecoup insults result in a focal contusion at the site and opposite site of impact, respectively. Direct head impact produces a focal cerebral contusion. Subsequent acceleration of the brain to the opposite side of the skull then results in a similar lesion opposite to the initial impact (Figure 2) [8]. Several other proposed mechanisms that likely contribute to the injury process exist. A study with balloons filled with fluids of different densities mimicking the brain and cerebral spinal fluid (CSF) suggest that the denser CSF shifts toward the impact site and displaces the brain to the opposite side of the skull [9]. Not surprisingly therefore, the contrecoup injury represents the initial impact of the brain with the skull and explains why the contrecoup injury site is often more severe [10]. Positive pressure theory suggests that contrecoup injury is a result of the brain lagging behind the skull and compressing against the opposite side of injury during the initial movement [11]. The negative pressure and shear stress theories on the other hand suggest that axial and rotational brain movements directly causing damage, respectively [12].
Figure 2: Coup-Contrecoup Injury.
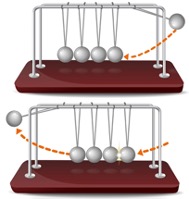
Helmets have largely proved successful in preventing skull fractures from primary and secondary impacts. However, they do not similarly protect against closed head injury, including focal contusions, hematomas, and concussions. The distribution of energy across the helmet and skull prevents fracture but may not adequately dissipate energy transmission affecting the brain [13]. Newton’s cradle is a device that uses swinging spheres in series to demonstrate the conservation of momentum and kinetic energy (Figure 3). Force is transmitted through the middle spheres, which remain stationary. Molecules partially connected by electrostatic forces constrain each other and transfer the pressure wave to the last sphere. This transfer can occur when a dense object is immersed in a less dense medium [14]. For example, in lithotripsy, shock waves can destroy kidney stones without the injuring intermittent structures. An ideal system would be one of elastic collision in which the head and brain transfer but do not absorb energy. A helmet with a soft outer layer covering the hard inner layer may take advantage of this concept. The soft layer may reduce the initial severity and facilitate the transfer of energy as seen in Newton’s cradle. Such helmets have been developed, though their efficacy is unknown, representing an opportunity for future research.
Figure 3: Newton’s Cradle.
Macroscopic Approaches for Preventing TBI
The pathophysiology of TBI is complex and mitigated by various mechanisms that are interconnected; however, analysis of these mechanisms and their commensurate prevention may be simplified by examination through categorization of the pathophysiology into macroscopic, microscopic, and molecular causes. Macroscopically, TBI is mediated by direct force to the head as well as differences in the physical mechanics related to the components of the central nervous system and its surrounding structures [8,15]. Within the skull, there is the brain, CSF, and a system of vascular structures, which serves to perfuse the brain through various arteries. The system drains through a series of venous structures located within dura [8,16,17]. As the skull is an inelastic, rigid structure, interaction with the brain may cause mechanical injury [8]. Therefore, the skull is filled with a complex network of CSF, which circulates within the ventricular system and, notably, is present within the subarachnoid space. The CSF functions to prevent mechanical injury to the brain by acting as a buffer to absorb shock [16,17]. However, the suspension of the brain in CSF confers freedom of movement of the brain within the skull, which may also lead to mechanical injury [15]. For example, direct force to the head may cause a cerebral contusion and acceleration of the brain within the skull [8]. This aforementioned acceleration then may cause the brain to impact the side of the skull opposite to the injury [8,15,18]. Together, these injuries are known as, ‘coup contrecoup injury’ [17], and the mechanism of injury through which dynamic forces cause movement of the brain and its fluids within the skull is known as ‘slosh’ [6,15,19]. As most conventional protective methods, such as helmets, protect the skull but fail to mitigate the brain’s freedom of movement, slosh injury is not addressed by these conventional methods [15,19]. To properly address slosh injury, protective methods must focus on the freedom of movement of the brain within the skull.
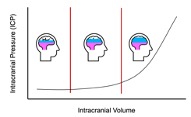
Increases in intracranial volume cause a reduction in intracranial compliance thereby reducing the brain’s movement within the skull, or slosh, and risk of TBI [6,15,19]. Of the components within the skull (the brain, CSF, and blood), blood is the component that is acquiescent to rapid changes in volume and, thereby pressure [15]. Therefore, an increase in cerebral blood volume would allow a reduction in intracranial compliance and confer protection to TBI through reduced intracranial collisions [6,15]. As previously mentioned, the cerebral vasculature drains through a series of venous channels with primary outflow via the IJV [16]. Constriction of the IJV alters intracerebral venous hemodynamics by reducing cerebral venous drainage, which increases intracranial volume as evidenced by an increase in intracranial pressure (ICP) and intraocular pressure (IOP) [20,21]. This is similar to the previously described Queckenstedt maneuver, where compression of the bilateral IJVs results in increased ICP [6]. Additionally, it is important to note the relationship between the intracranial volume and ICP as described by the Monro-Kellie doctrine (Figure 4) [22].
Figure 4: The skull confines the brain, cerebrospinal fluid, and intracranial blood. If volume increases in any of these dynamics, the system eventually loses ability to compensate and intracranial pressure peaks.
To assess the effect of IJV constriction, a rat model used a compressive collar to induce constriction of the IJV, which resulted in over a 30% increase in ICP and IOP within seconds [15]. Additionally, rats equipped with the compressive collar showed a reduced severity of TBI injury, as evidenced by the number of amyloid precursor protein-positive (APP-positive) neurons on autopsy: p <0.01 [15]. In a similar rat model, collar-induced IJV compression resulted in a 48.7%-59.1% reduction in degenerative neurons as evidenced by fluoro-jade B (FJB), a 36.8%–45.7% decrease in reactive astrocytes as measured by glial fibrillary acidic protein (GFAP), and a 44.1%–65.3% reduction in microglial activation as evidenced by ionized calcium-binding adapter molecule 1 (IBA1) [6]. This cerebral protection through venous constriction and resulting increased IOP is also found to be a protective element of woodpeckers [6,15,23].
Furthermore, a similar mechanism may be capable in humans [6,15,19]. In humans, the IJV passes between the two muscle bellies of the omohyoid [6,24]. Therefore, constriction of the IJV by contraction of the omohyoid may result in this same alteration of intracerebral hemodynamics and protection from TBI [6,15]. Some researchers hypothesize that professional boxers are able to withstand expected blows much greater than unexpected by the contraction of the neck muscles, including the omohyoid [6]. Other research has demonstrated that muscle contraction prior to impact reduces head kinematics [25,26], and that neck strength is a protective factor against TBI [25-27]. This protective effect is likely related to biomechanical protection against head acceleration but may also include protective effects caused by neck muscle constriction of the IJV. To enhance the protective effect, IJV compressive collars have been examined in American Football and shown to reduce white matter changes after repetitive head impacts [28,29]. Similar jugular collars have also demonstrated the protective effect against slosh injury in military and police training during explosives training [30-32].
Microscopic Approaches for Preventing TBI
In nature, there are several animals that withstand repeated head impacts of significant force without resultant long standing injury. Birds and Bighorn sheep may both have developed unique respiratory adaptations that allow for increased carbon dioxide (CO2) mediated cerebral blood flow alterations that have a neuroprotective effect during impacts.
The avian respiratory system is a unique and efficient system of gas exchange, delivering oxygen to tissue and removing CO2 from blood. Physically distinct from the mammalian respiratory system it utilizes nine air sacs and a pair of lungs. Birds have two cervical air sacs, an unpaired clavicular sac, two cranial thoracic sacs, two caudal thoracic sacs, and two abdominal air sacs (Figure 5). The sacs are arranged into two groups, those coming off the anterior of the lungs and those arising from the posterior of the lungs. These thin-walled structures are composed of simple squamous epithelium, a thin layer of connective tissue and blood vessels. Unlike other vertebrates whose lungs allow for bidirectional flow of air due to expansion and contraction, the avian lungs are static with the air sacs functioning as bellows that expand and contract, forcing air though the lungs in a unidirectional fashion [33].
Figure 5: Overview of avian lung sacs.
The respiration of birds consists of two cycles of inhalation and exhalation. In the first inhalation, air enters through the nares, goes down the trachea and into each primary bronchus. Some enters the lungs and participates in the exchange of oxygen and CO2 through the air-capillary system, while the remainder enters the posterior sacs. When the bird exhales, the fresh air in the posterior sacs then enters the lungs, displacing the spent air. Thereby the process facilitates gas exchange. The spent air exits through the trachea. During the second inhalation, the air again enters through the trachea into the posterior sacs and lungs, displacing the spent air, but cannot yet exit through the trachea due to the inward flow of air. Instead, the CO2 rich air enters the anterior sacs. During the second exhalation, air from the lungs and anterior sacs is expelled through the trachea [33].
Additionally, the respiratory sacs invade the bone via diverticula, thus pneumatizing them. When the diverticula come into contact with each other, they may unite, creating a continuous airway from the sacs into the bone. Pneumatic bones are common in the skull, humerus, clavicle, keel (sternum), pelvic girdle, and the lumbar and sacral vertebrae in birds that fly [33].
Bighorn sheep possess horns that are made up of an outer sheath that encases a bone core called the horncore. The outer sheath is made of keratin, the protein found in human hair and nails, and is organized into a protein-based matrix of lamellar sheets [7]. These sheets form hollow, elliptical tubules dispersed between layers that extend and grow forming the horn. Myers et al. has suggested that the horns of the bighorn sheep are themselves pneumatic organs, continuous with the respiratory system, thereby allowing the animal to rebreathe its air to increase the carbon dioxide within its blood. The increase in the partial pressure of CO2 would induce vasodilation and subsequent increased intracranial volume, creating a tighter fit of the brain inside the cranium, reducing slosh, and thus, brain injury [34]
CO2 is a mediator of cerebral blood flow (CBF), and this effect has been well studied. Physicians first started to explore the use of hyperventilation to lower cerebral blood volume and ‘ICP during the 1920s [35]. CO2 concentration is a key determinant of intracranial volume and elevated arterial CO2 tension leads to a reversible relaxation and dilation of cerebral arteries and arterioles and increased CBF, whereas hypocapnia causes constriction and decreased blood flow [36]. The vasodilator effects of CO2 in humans are demonstrated by the observation that inhalation of 5% CO2 results in an 50% increase in CBF, and 7% CO2 inhalation causes a 100% increase in CBF [37]. Cerebral blood volume is also impacted by alterations in the partial pressure of CO2, but the relative change is less marked than CBF [38]. CO2 is not only a potent dilator of cerebral vasculature; it is also an effective ocular vasodilator of retinal and optic nerve blood flow [39].
Given the potential physiologic explanations for the observed protection from brain injury in animals such as the hummingbird and bighorn sheep, it is no surprise that the search continues for a similar approach to manipulate CO2 in humans. By increasing the partial pressure of CO2, we may induce cerebral vasodilation and lead to increased ICP, thus reducing the shearing and cavitation caused by rapid acceleration/deceleration that are the hallmark of slosh-injury [34]. Increased partial pressure of CO2 or hypercapnia not only mitigates macro-slosh injury, it also may also reduce molecular slosh of hemoglobin molecules present within red blood cells by reducing the absorption of forces by the tissue through an increase in the elasticity of collisions. Hypercapnia may be induced by a respiratory circuit which could be a breathing or non-breathing circuit mask, or a breathing circuit capable of controlling the amount of inhaled CO2 (Figures 6 and 7).
Figure 6: Partial rebreather mask that could be used to increase CO2 in humans.
Figure 7: Respirator device that can acutely increase venous CO2.
Because of the interplay between intracranial volume, partial pressure of CO2 and severity of TBI, it was suspected by Smith et al. that the physiologic acclimatization to increased altitude may lead to changes within the cranium that would reduce the rate off concussion experienced by high school athletes. These changes include increased ICP and would be a result of hypoxic vasogenic edema. They showed that among athletes playing at a higher altitude, there was a 31% reduction in the incidence of total reported concussions and 30% decrease in concussion rate for overall exposures, 27% for competition exposures, and 28% for practice exposures in football players. This study included nearly 6000 athletes from almost 500 schools with locations ranging from 2.1-2104 meters above sea level [40]. A follow up to this epidemiologic study was conducted by Myer and Smith et al. amongst National Football League (NFL) players, hypothesizing that games played at a higher elevation would have a lower rate of concussion than games played at a lower elevation. They found that the rate of concussion was 30% lower in games played at or above 196.3 meters. These results show that like bighorn sheep and other animals, a protective effect of hypercapnia induced increase in intracranial blood volume may be present in humans, albeit to a smaller degree [34].
Molecular Approaches for Preventing TBI
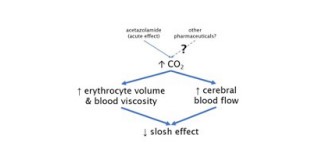
Targeted molecular modifications affecting erythrocyte membrane properties, hemoglobin structure, and blood viscosity are promising avenues to reduce TBI [41]. These therapeutic approaches are hypothesized to act via modulation of intracranial compliance, thus minimizing the slosh effect. Erythrocytes cannot execute typical cellular repair processes like a nucleated cell; thus, many molecular alterations last the lifetime of the cell. After TBI, one of the primary injuries to erythrocytes is compromised cell membrane function [42]. Animal models of TBI have revealed an increase in markers of lipid peroxidation in RBCs as well as increased aggregation and decreased electrophoretic mobility, both markers of RBC function and membrane distensibility. Experiments with isolated human blood have shown similar trends; blast waves transmitted through steel containers cause lysis of erythrocytic cell membranes but can be mitigated by decreasing free space within the container to reduce the slosh effect [43]. Translation of this principle into in vivo systems could be achieved with interventions which increase erythrocyte volume or blood viscosity (Figure 8).
Figure 8: Mechanisms that increase CO2, erythrocyte volume, and/or cerebral blood flow can have protective effects on the ‘slosh’ effect
As discussed in previous sections, the partial pressure of CO2 is a critical mediator of cerebral autoregulation as well as erythrocyte function [44,45]. Hypercapnia causes a right shift in the oxyhemoglobin dissociation curve, thus increasing O2 off-loading in hypoxic tissues. Further, increased pCO2 increases RBC volume through accumulation of bicarbonate, chloride, hydrogen, and other ions [46,47]. This effect serves to increase the viscosity of blood by increasing the proportion of total volume held within the cellular compartment [48]. Increased blood viscosity along with increased cerebral blood flow may be the crucial factors explaining the observation of fewer concussions during football games played at higher altitudes [34,40]. These factors are also consistent with the hypotheses explaining the innate neuroprotection seen in Bighorn Sheep. Hypercapnia induced by altitude and their unique respiratory anatomy allows Bighorn Sheep to minimize intracranial compliance and reduce the fluid slosh effect.
Pharmacologic modulation of erythrocyte function and total cerebral blood flow with the carbonic anhydrase acetazolamide could leverage these principles to prevent TBI (Table 1). Single doses of acetazolamide are sufficient to increase cerebral blood flow [49], which could potentially beneficial in reducing brain slosh although this remains to be tested directly. Another study demonstrated acetazolamide treatment prior to exercise resulted in elevated arterial and venous CO2 partial pressures [50], which may impart all of the benefits of hypercapnia on brain dynamics during TBI. More investigation in disease-specific contexts is needed to fully evaluate the therapeutic potential of acetazolamide in TBI prevention.
|
References |
Study Design |
Critical Observations |
|
Erythrocyte Disruption after TBI |
||
|
41 |
Randomized trial in a rat model of TBI |
TBI causes increased erythrocytic lipid peroxidation and increases erythrocyte aggregation. |
|
42 |
ex vivo analysis of human blood subjected to simulated explosive blast waves |
Decreasing free space within a container (no slosh) reduces erythrocyte disruption and hemolysis compared to incompletely filled containers (slosh). |
|
Effect of CO2 on erythrocytes & CBF |
||
|
47 |
ex vivo analysis of whole blood from multiple mammal species |
Erythrocytes increased in volume proportional to the relative CO2 content of blood. |
|
48 |
ex vivo analysis of human blood |
Increasing pCO2 causes an increase in blood viscosity |
|
44 |
Non-controlled clinical trial |
Transient hyperventilation is associated with increased cerebral autoregulation reactivity. |
|
50 |
Randomized trial in horses |
Acetazolamide increases arterial and venous pCO2 during exercise without significantly affecting oxygenation. |
|
Human Observational Studies & Pharmacologic Approaches |
||
|
49 |
Non-controlled clinical trial |
Single dose of acetazolamide increases CBF by ~40%. |
|
19 |
Observational studies of amateur and professional athletes |
Altitude is inversely associated with risk of concussion during American football games |
TBI & Hearing Loss: A Shared Mechanism
The association of TBI of the blast variety with hearing loss is well established [51], Within the subset of TBI patients, Lew et al. noted that those with blast-related TBI have significantly higher rates of hearing loss than those with non-blast-related TBI (62% vs 44%, p=0.04) [52]. Barotrauma-mediated tympanic membrane rupture is one well-characterized mechanism of hearing loss following blast exposure [53]. However, slosh-mediated damage to anatomical structures involved in audition may be another mechanism for hearing loss following blast exposure. Cochlear hair cells may be particularly susceptible to slosh-mediated damage in the setting of blast-related TBI, due to their relative fragility and mobility in the inner ear. Thus, mitigating slosh may help prevent damage to these structures during blast-related TBI. One proposed mechanism of slosh-mitigation in this setting is increasing intracranial volume via cranial venous sinus engorgement secondary to jugular vein compression. Increasing intracranial volume may be transmitted to the inner ear space, increasing volume and pressure surrounding the hair cells and attenuating slosh-mediated damage during a blast by reducing relative mobility of hair cells [31]. Other mechanisms involving redirecting energy from blasts may be similarly beneficial in mediating slosh-associated damage to anatomical structures and subsequently minimizing hearing loss.
Investigation of Slosh: A Way Forward
TBI is linked to cerebral edema, blood-brain barrier disruption, and neuroinflammation, all of which play a role in the severity of the injury and functional recovery. Recent advancements in TBI research imply that slosh mitigation, a method of limiting the brain's ability to move inside the skull in both linear and rotational directions, may have the potential to prevent TBI [6,15,43]. The invention of a moderate jugular compression collar to enhance the resistance of the jugular vascular tree evolved from a better understanding of the mechanics of slosh as it applies to blood within the cranium [6]. More blood was hypothesized to be diverted to the vertebral veins (increasing CBF) and other capacitance arteries with higher vascular resistance, which occupied cerebral space and limited potential mobility [6,15,43]. Research studies have revealed that IJV compression significantly lowers signs of neurological injury, as measured by FJB levels and posttraumatic glial activation, with substantial reductions in the cortex, hippocampus, striatum, and cerebellum [6].
Other treatments have now emerged that follow the similar ideas of enhancing cerebral blood flow and cerebral perfusion pressure in the hopes of limiting brain impairment after a TBI by employing the principles of slosh mitigation. The strategies, to prevent subsequent injury after a TBI, range from postural adjustments to pharmacological interventions that can maintain cranial pressures, brain tissue perfusion, and reduce inflammation. Head immobilization and therapeutic positioning of the head (varying degrees of head of bed elevation (HBE)) have been advocated as a low-cost and straightforward method of preventing subsequent brain injury [54-57]. In a study by Winkelman et al., HBE of 30° has been reported to be a therapeutic technique to consider for preventing elevated ICP in TBI patients [55]. Other studies, on the other hand, have been unable to reach a consensus on therapeutic impact of HBE of 30°, and thus suggest that the optimum angle of HBE should be determined individually, with the desired clinical goal in mind, and after an analysis of the response of ICP, cerebral perfusion pressure (CPP), and CBF in each backrest positions [54-57].
Monitoring ICP in individuals with TBI is critical for preventing further damage. An elevation in ICP is associated with a drop in CPP and, as a result, a decrease in CBF, which can lead to secondary ischemic phenomena. Hypocapnia is hypothesized to cause cerebral vasoconstriction and is commonly used to regulate ICP but chronic hypocapnia in TBI patients increases the risk of death and severe disability [58-60]. Mild hypercapnia, on the other hand, may enhance CBF by cerebral vasodilation, which has a therapeutic impact in cerebral ischemia following a TBI [58,59,61]. TBI is also known to cause cerebral bleeding and RBC lysis, which causes Hb and heme to be released and absorbed by microglia and neurons [62,63]. This causes oxidative damage and inflammation in brain tissue, which can lead to cytotoxic edema [63-65]. Acetazolamide can target one of the molecular targets connected to cytotoxic edema, AQP4, in the astrocyte. In an in vivo mouse TBI model, acetazolamide was shown to reduce cytotoxic edema [64,66]. Another study looked at the effectiveness of the antioxidant methylene blue (MB) in decreasing inflammation and behavioral problems associated with diffuse brain damage [67]. This is especially noteworthy because antioxidant levels have been found to drop in both adult and pediatric TBI patients across several studies [65,67]. Hence, MB intervention is a good potential therapeutic approach that may reduce life-threatening complications of TBI, such as cerebral edema and neuroinflammation, and protect against the development of secondary and long-term neuropsychiatric complications [64-67].
After an injury, the brain’s ability to pressure autoregulate may be compromised, and CBF might passively follow shifts in CPP [6,15,43,54,60,61]. To maintain optimal blood flow and perfusion, it is critical to reduce inflammation, edema, and oxidative stress in the brain. In animal TBI models, the protective effects of Minocycline (a microglia activator) and DFX (an iron chelator) on protecting BBB and lowering inflammation through decreasing TNF-alpha and suppressing oxidative stress to avoid edema have been explored [68-70].
Conclusion
Further prospective studies are needed to determine the efficacy of the aforementioned strategies, as well as other theorized methodologies, in preventing and managing TBI patients. This could lead to new insights into therapies that can significantly reduce subsequent insult/injury after a TBI. Looking forward, slosh mitigation and early neuroprotective interventions, as opposed to extracranial protective equipment like helmets, appear to offer a fresh paradigm for preventing and managing TBI by successfully targeting the intracranial environment.
References
2. Liu Y, Qiu X, Zhang X, Yu TX. Response of woodpecker's head during pecking process simulated by Material Point Method. PloS one. 2015 Apr 22;10(4):e0122677.
3. Weisberger M. Does All That Headbanging Leave a Mark on Woodpeckers' Brains? Live Science: Future US, Inc; 2018.
4. Chang B, Croson M, Straker L, Gart S, Dove C, Gerwin J, et al. How seabirds plunge-dive without injuries. Proceedings of the National Academy of Sciences. 2016 Oct 25;113(43):12006-11.
5. Aguirre TG, Fuller L, Ingrole A, Seek TW, Wheatley BB, Steineman BD, et al. Bioinspired material architectures from bighorn sheep horncore velar bone for impact loading applications. Scientific Reports. 2020 Nov 3;10(1):1-4.
6. Turner RC, Naser ZJ, Bailes JE, Smith DW, Fisher JA, Rosen CL. Effect of slosh mitigation on histologic markers of traumatic brain injury. Journal of Neurosurgery. 2012 Dec 1;117(6):1110-8.
7. Drake A, Donahue TL, Stansloski M, Fox K, Wheatley BB, Donahue SW. Horn and horn core trabecular bone of bighorn sheep rams absorbs impact energy and reduces brain cavity accelerations during high impact ramming of the skull. Acta Biomaterialia. 2016 Oct 15;44:41-50.
8. Davis AE. Mechanisms of traumatic brain injury: biomechanical, structural and cellular considerations. Critical Care Nursing Quarterly. 2000 Nov 1;23(3):1-3.
9. Drew LB, Drew WE. The contrecoup-coup phenomenon. Neurocritical Care. 2004 Sep;1(3):385-90.
10. Payne WN, De Jesus O, Payne AN. Contrecoup Brain Injury. StatPearls Publishing LLC. 2021. Treasure Island (FL), United States.
11. Goggio AF. The mechanism of contre-coup injury. Journal of Neurology and Psychiatry. 1941 Jan;4(1):11.
12. Allen FJ. The Mechanism of Contre-Coup and of Certain Other Forms of Intracranial Injury. British Medical Journal. 1896 May 16;1(1846):1196.
13. Rughani AI, Lin CT, Ares WJ, Cushing DA, Horgan MA, Tranmer BI, et al. Helmet use and reduction in skull fractures in skiers and snowboarders admitted to the hospital. Journal of Neurosurgery: Pediatrics. 2011 Mar 1;7(3):268-71.
14. Herrmann F, Seitz M. How does the ball‐chain work? American Journal of Physics. 1982 Nov;50(11):977-81.
15. Smith DW, Bailes JE, Fisher JA, Robles J, Turner RC, Mills JD. Internal jugular vein compression mitigates traumatic axonal injury in a rat model by reducing the intracranial slosh effect. Neurosurgery. 2012 Mar 1;70(3):740-6.
16. Chandra A, Li WA, Stone CR, Geng X, Ding Y. The cerebral circulation and cerebrovascular disease I: Anatomy. Brain Circulation. 2017 Apr;3(2):45.
17. Wright BL, Lai JT, Sinclair AJ. Cerebrospinal fluid and lumbar puncture: a practical review. Journal of Neurology. 2012 Aug;259(8):1530-45.
18. Rungruangsak K, Poriswanish N. Pathology of fatal diffuse brain injury in severe non-penetrating head trauma. Journal of Forensic and Legal Medicine. 2021 Aug 1;82:102226.
19. Myer GD, Yuan W, Barber Foss KD, Smith D, Altaye M, Reches A, et al. The effects of external jugular compression applied during head impact exposure on longitudinal changes in brain neuroanatomical and neurophysiological biomarkers: a preliminary investigation. Frontiers in Neurology. 2016 Jun 6;7:74.
20. Raphael JH, Chotai R. Effects of the cervical collar on cerebrospinal fluid pressure. Anaesthesia. 1994 May;49(5):437-9.
21. Teng C, Gurses-Ozden R, Liebmann JM, Tello C, Ritch R. Effect of a tight necktie on intraocular pressure. British Journal of Ophthalmology. 2003 Aug 1;87(8):946-8.
22. Rabelo NN, da Silva Brito J, da Silva JS, de Souza NB, Coelho G, Brasil S, et al. The historic evolution of intracranial pressure and cerebrospinal fluid pulse pressure concepts: Two centuries of challenges. Surgical Neurology International. 2021;12.
23. Wang L, Cheung JT, Pu F, Li D, Zhang M, Fan Y. Why do woodpeckers resist head impact injury: A Biomechanical Investigation. PloS one. 2011 Oct 26;6(10):e26490.
24. Rai R, Ranade A, Nayak S, Vadgaonkar R, Mangala P, Krishnamurthy A. A study of anatomical variability of the omohyoid muscle and its clinical relevance. Clinics. 2008;63(4):521-4.
25. Collins CL, Fletcher EN, Fields SK, Kluchurosky L, Rohrkemper MK, Comstock RD, et al. Neck strength: a protective factor reducing risk for concussion in high school sports. The Journal of Primary Prevention. 2014 Oct 1;35(5):309-19.
26. Eckner JT, Oh YK, Joshi MS, Richardson JK, Ashton-Miller JA. Effect of neck muscle strength and anticipatory cervical muscle activation on the kinematic response of the head to impulsive loads. The American Journal of Sports Medicine. 2014 Mar;42(3):566-76.
27. Elliott J, Heron N, Versteegh T, Gilchrist IA, Webb M, Archbold P, et al. Injury reduction programs for reducing the incidence of sport-related head and neck injuries including concussion: a systematic review. Sports Medicine. 2021 Jun 18:1-6.
28. Myer GD, Yuan W, Foss KD, Thomas S, Smith D, Leach J, et al. Analysis of head impact exposure and brain microstructure response in a season-long application of a jugular vein compression collar: a prospective, neuroimaging investigation in American football. British Journal of Sports Medicine. 2016 Oct 1;50(20):1276-85.
29. Yuan W, Barber Foss KD, Thomas S, DiCesare CA, Dudley JA, Kitchen K, et al. White matter alterations over the course of two consecutive high‐school football seasons and the effect of a jugular compression collar: A preliminary longitudinal diffusion tensor imaging study. Human Brain Mapping. 2018 Jan;39(1):491-508.
30. Bonnette S, Diekfuss JA, Kiefer AW, Riley MA, Foss KD, Thomas S, et al. A jugular vein compression collar prevents alterations of endogenous electrocortical dynamics following blast exposure during special weapons and tactical (SWAT) breacher training. Experimental Brain Research. 2018 Oct;236(10):2691-701.
31. Yuan W, Barber Foss KD, Dudley J, Thomas S, Galloway R, DiCesare C, et al. Impact of low-level blast exposure on brain function after a one-day tactile training and the ameliorating effect of a jugular vein compression neck collar device. Journal of Neurotrauma. 2019 Mar 1;36(5):721-34.
32. Yuan W, Dudley J, Slutsky-Ganesh AB, Leach J, Scheifele P, Altaye M, et al. White matter alteration following SWAT explosive breaching training and the moderating effect of a neck collar device: a DTI and NODDI study. Military Medicine. 2021 May 3.
33. Gill FB. Ornithology at a Centennial: Perspectives in Ornithology. Essays Presented for the Centennial of the American Ornithologists' Union. Alan H. Brush and George A. Clark, Jr., Eds. Cambridge University Press, New York, 1983. viii, 560 pp., illus. $29.95. Science. 1984 Feb 17;223(4637):693-.
34. Myer GD, Smith D, Barber Foss KD, Dicesare CA, Kiefer AW, Kushner AM, et al. Rates of concussion are lower in National Football League games played at higher altitudes. Journal of Orthopaedic & Sports Physical Therapy. 2014 Mar;44(3):164-72.
35. Diringer M. Hyperventilation in head injury: What have we learned in 43 years?. Critical Care Medicine. 2002 Sep 1;30(9):2142-3.
36. Cipolla MJ. Control of cerebral blood flow. InThe cerebral circulation 2009. Morgan & Claypool Life Sciences.
37. Kety SS, Schmidt CF. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. The Journal of Clinical Investigation. 1948 Jul 1;27(4):484-92.
38. Brian JE. Carbon dioxide and the cerebral circulation. The Journal of the American Society of Anesthesiologists. 1998 May 1;88(5):1365-86.
39. Hvidberg A, KESSING SV, Fernandes A. Effect of changes in PC02 and body positions on intraocular pressure during general anaesthesia. Acta Ophthalmologica. 1981 Aug;59(4):465-75.
40. Smith DW, Myer GD, Currie DW, Comstock RD, Clark JF, Bailes JE. Altitude modulates concussion incidence: implications for optimizing brain compliance to prevent brain injury in athletes. Orthopaedic Journal of Sports Medicine. 2013 Nov 8;1(6):2325967113511588.
41. Polozova Anastasia V, Boyarinov Gennadii A, Nikolsky Viktor O, Zolotova Marina V, Deryugina Anna V. The functional indexes of RBCs and microcirculation in the traumatic brain injury with the action of 2-ethil-6-methil-3-hydroxypiridin succinate. BMC Neuroscience. 2021 Dec;22(1):1-5.
42. Smith JE, Garner J. Pathophysiology of primary blast injury. BMJ Military Health. 2019 Feb 1;165(1):57-62.
43. Smith MM, Renew JR, Nelson JA, Barbara DW. Red blood cell disorders: Perioperative considerations for patients undergoing cardiac surgery. Journal of Cardiothoracic and Vascular Anesthesia. 2019 May 1;33(5):1393-406.
44. Zhang Y, Liu X, Steiner L, Smielewski P, Feen E, Pickard JD, et al. Correlation between cerebral autoregulation and carbon dioxide reactivity in patients with traumatic brain injury. InIntracranial Pressure and Brain Monitoring XV 2016 (pp. 205-209). Springer, Cham.
45. Wang YZ, Li TT, Cao HL, Yang WC. Recent advances in the neuroprotective effects of medical gases. Medical Gas Research. 2019 Apr;9(2):80.
46. Takiwaki H, Mochizuki M, Niizeki K. Relationship between hematocrit and CO2 contents in whole blood and true plasma. The Japanese Journal of Physiology. 1983;33(4):567-78.
47. Jackson DM, Nutt ME. The effect of carbon dioxide on relative red cell volume. The Journal of Physiology. 1954 Feb 26;123(2):367.
48. Solvsteen P, Kristjansen PF. The Effect of Carbon Dioxide and Oxygen on the Viscosity of Whole Blood. Zeitschrift Fur Kreislaufforschung. 1968 Jan;57(1):42-6.
49. Friberg LA, Kastrup JE, Rizzi DO, Jensen JB, Lassen NA. Cerebral blood flow and end-tidal PCO2 during prolonged acetazolamide treatment in humans. American Journal of Physiology-Heart and Circulatory Physiology. 1990 Apr 1;258(4):H954-9.
50. Vengust M, Staempfli H, Viel L, Swenson ER, Heigenhauser G. Acetazolamide attenuates transvascular fluid flux in equine lungs during intense exercise. The Journal of Physiology. 2013 Sep;591(18):4499-513.
51. Fausti SA, Wilmington DJ, Gallun FJ, Myers PJ, Henry JA. Auditory and vestibular dysfunction associated with blast-related traumatic brain injury. Journal of Rehabilitation Research & Development. 2009 Jul 1;46(6).
52. Lew HL, Jerger JF, Guillory SB, Henry JA. Auditory dysfunction in traumatic brain injury. Journal of Rehabilitation Research & Development. 2007 Dec 1;44(7).
53. Belanger HG, Proctor-Weber Z, Kretzmer T, Kim M, French LM, Vanderploeg RD. Symptom complaints following reports of blast versus non-blast mild TBI: does mechanism of injury matter?. The Clinical Neuropsychologist. 2011 Jul 1;25(5):702-15.
54. Winkelman C. Effect of backrest position on intracranial and cerebral perfusion pressures in traumatically brain-injured adults. American Journal of Critical Care. 2000 Nov 1;9(6):373.
55. Alarcon JD, Rubiano AM, Okonkwo DO, Alarcón J, Martinez‐Zapata MJ, Urrútia G, et al. Elevation of the head during intensive care management in people with severe traumatic brain injury. Cochrane Database of Systematic Reviews. 2017(12).
56. March K, Mitchell P, Grady S, Winn R. Effect of backrest position on intracranial and cerebral perfusion pressures. The Journal of neuroscience nursing: Journal of the American Association of Neuroscience Nurses. 1990 Dec 1;22(6):375-81.
57. Meixensberger J, Baunach S, Amschler J, Dings J, Roosen K. Influence of body position on tissue-p O2, cerebral perfusion pressure and intracranial pressure in patients with acute brain injury. Neurological Research. 1997 Jun 1;19(3):249-53.
58. Deng RM, Liu YC, Li JQ, Xu JG, Chen G. The role of carbon dioxide in acute brain injury. Medical Gas Research. 2020 Apr;10(2):81.
59. Martindale T, McGlone P, Chambers R, Fennell J. Management of severe traumatic brain injury and acute respiratory distress syndrome using pumped extracorporeal carbon dioxide removal device. Journal of the Intensive Care Society. 2017 Feb;18(1):66-70.
60. Balestreri M, Czosnyka M, Hutchinson P, Steiner LA, Hiler M, Smielewski P, et al. Impact of intracranial pressure and cerebral perfusion pressure on severe disability and mortality after head injury. Neurocritical Care. 2006 Feb;4(1):8-13.
61. Bogossian EG, Peluso L, Creteur J, Taccone FS. Hyperventilation in adult TBI patients: How to approach it?. Frontiers in Neurology. 2020;11.
62. Go SL, Singh JM. Pro/con debate: Should PaCO2 be tightly controlled in all patients with acute brain injuries?. Critical Care. 2013 Feb;17(1):1-7.
63. Ziai WC. Hematology and inflammatory signaling of Intracerebral Hemorrhage. Stroke. 2013 Jun;44(6_suppl_1):S74-8.
64. Zheng H, Chen C, Zhang J, Hu Z. Mechanism and therapy of brain edema after intracerebral hemorrhage. Cerebrovascular Diseases. 2016;42(3-4):155-69.
65. Bayir H, Kagan VE, Tyurina YY, Tyurin V, Ruppel RA, Adelson PD, et al. Assessment of antioxidant reserves and oxidative stress in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatric Research. 2002 May;51(5):571-8.
66. Glober NK, Sprague S, Ahmad S, Mayfield KG, Fletcher LM, Digicaylioglu MH, et al. Acetazolamide treatment prevents redistribution of astrocyte aquaporin 4 after murine traumatic brain injury. Neuroscience Journal. 2019;2019.
67. Fenn AM, Skendelas JP, Moussa DN, Muccigrosso MM, Popovich PG, Lifshitz J, et al. Methylene blue attenuates traumatic brain injury-associated neuroinflammation and acute depressive-like behavior in mice. Journal of Neurotrauma. 2015 Jan 15;32(2):127-38.
68. Gu Y, Hua Y, Keep RF, Morgenstern LB, Xi G. Deferoxamine reduces intracerebral hematoma-induced iron accumulation and neuronal death in Piglets. Stroke. 2009 Jun 1;40(6):2241-3.
69. Wu J, Yang S, Hua Y, Liu W, Keep RF, Xi G. Minocycline attenuates brain edema, brain atrophy and neurological deficits after Intracerebral Hemorrhage. InBrain Edema XIV 2010 (pp. 147-150). Springer, Vienna.
70. Xie Q, Gu Y, Hua Y, Liu W, Keep RF, Xi G. Deferoxamine attenuates white matter injury in a Piglet Intracerebral Hemorrhage model. Stroke. 2014 Jan;45(1):290-2.